- Record: found
- Abstract: found
- Article: found
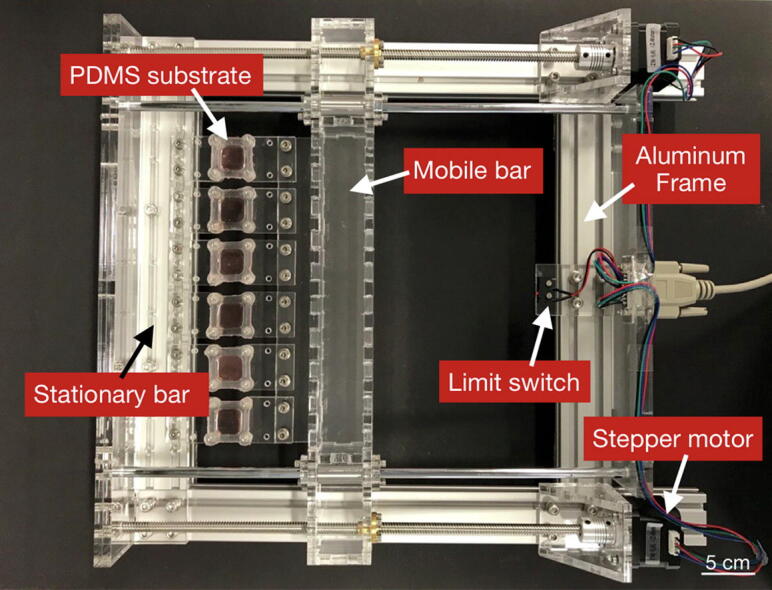
A low-cost uniaxial cell stretcher for six parallel wells
Read this article at
Graphical abstract
Abstract
Cells in the lungs, the heart, and numerous other organs, are constantly exposed to dynamic forces and deformations. To mimic these dynamic mechanical loading conditions and to study the resulting cellular responses such as morphological changes or the activation of biochemical signaling pathways, cells are typically seeded on flexible 2D substrates that are uniaxially or biaxially stretched. Here, we present an open-source cell stretcher built from parts of an Anet A8 3D printer. The cell stretcher is controlled by a fully programmable open-source software using GCode and Python. Up to six flexible optically clear substrates can be stretched simultaneously, allowing for comparative multi-batch biological studies including microscopic image analysis. The cell yield from the cell culture area of 4 cm 2 per substrate is sufficient for Western-blot protein analysis. As a proof-of-concept, we study the activation of the Yes-associated protein (YAP) mechanotransduction pathway in response to increased cytoskeletal tension induced by uniaxial stretching of epithelial cells. Our data support the previously observed activation of the YAP transcription pathway by stretch-induced increase in cytoskeletal tension and demonstrate the suitability of the cell stretcher to study complex mechano-biological processes.
Related collections
Most cited references40
- Record: found
- Abstract: found
- Article: not found
Role of YAP/TAZ in mechanotransduction.
- Record: found
- Abstract: found
- Article: not found
Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling.
- Record: found
- Abstract: found
- Article: not found