- Record: found
- Abstract: found
- Article: not found
Androgens Regulate Prostate Cancer Cell Growth via an AMPK-PGC-1α-Mediated Metabolic Switch
Read this article at
Abstract
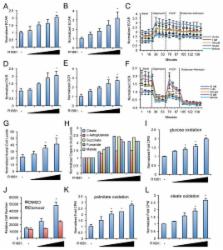
Prostate cancer is the most commonly diagnosed malignancy among men in industrialized countries, accounting for the second leading cause of cancer-related deaths. While we now know that the androgen receptor (AR) is important for progression to the deadly advanced stages of the disease, it is poorly understood what AR-regulated processes drive this pathology. Here, we demonstrate that AR regulates prostate cancer cell growth via the metabolic sensor 5′-AMP-activated protein kinase (AMPK), a kinase that classically regulates cellular energy homeostasis. In patients, activation of AMPK correlated with prostate cancer progression. Using a combination of radiolabeled assays and emerging metabolomic approaches, we also show that prostate cancer cells respond to androgen treatment by increasing not only rates of glycolysis, as is commonly seen in many cancers, but also glucose and fatty acid oxidation. Importantly, this effect was dependent on androgen-mediated AMPK activity. Our results further indicate that the AMPK-mediated metabolic changes increased intracellular ATP levels and peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α)-mediated mitochondrial biogenesis, affording distinct growth advantages to the prostate cancer cells. Correspondingly, we used outlier analysis to determine that PGC-1α is overexpressed in a subpopulation of clinical cancer samples. This was in contrast to what was observed in immortalized benign human prostate cells and a testosterone-induced rat model of benign prostatic hyperplasia. Taken together, our findings converge to demonstrate that androgens can co-opt the AMPK-PGC-1α signaling cascade, a known homeostatic mechanism, to increase prostate cancer cell growth. The current study points to the potential utility of developing metabolic-targeted therapies directed towards the AMPK-PGC-1α signaling axis for the treatment of prostate cancer.
Related collections
Most cited references46
- Record: found
- Abstract: found
- Article: not found
AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha.
- Record: found
- Abstract: found
- Article: not found
