- Record: found
- Abstract: found
- Article: found
A long-term "memory" of HIF induction in response to chronic mild decreased oxygen after oxygen normalization
Read this article at
Abstract
Background
Endothelial dysfunction (ED) is functionally characterized by decreased vasorelaxation, increased thrombosis, increased inflammation, and altered angiogenic potential, has been intimately associated with the progression and severity of cardiovascular disease. Patients with compromised cardiac function oftentimes have a state of chronic mild decreased oxygen at the level of the vasculature and organs, which has been shown to exacerbate ED. Hypoxia inducible factor (HIF) is a transcription factor complex shown to be the master regulator of the cellular response to decreased oxygen levels and many HIF target genes have been shown to be associated with ED.
Methods
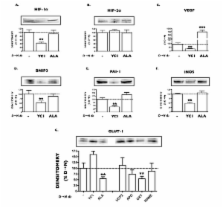
Human endothelial and aortic smooth muscle cells were exposed either to A) normoxia (21% O 2) for three weeks, or to B) mild decreased oxygen (15% O 2) for three weeks to mimic blood oxygen levels in patients with heart failure, or to C) mild decreased oxygen for two weeks followed by one week of normoxia ("memory" treatment). Levels of HIF signaling genes (HIF-1α, HIF-2α, VEGF, BNIP3, GLUT-1, PAI-1 and iNOS) were measured both at the protein and mRNA levels.
Results
It was found that chronic exposure to mild decreased oxygen resulted in significantly increased HIF signaling. There was also a "memory" of HIF-1α and HIF target gene induction when oxygen levels were normalized for one week, and this "memory" could be interrupted by adding a small molecule HIF inhibitor to the last week of normalized oxygen. Finally, levels of ubiquitylated HIF-1α were reduced in response to chronic mild decreased oxygen and were not full restored after oxygen normalization.
Related collections
Most cited references57
- Record: found
- Abstract: found
- Article: not found
HMEC-1: establishment of an immortalized human microvascular endothelial cell line.
- Record: found
- Abstract: found
- Article: not found