- Record: found
- Abstract: found
- Article: not found
ELISA for Determination of Human Growth Hormone: Recognition of Helix 4 Epitopes
Read this article at
Abstract
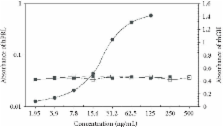
Human growth hormone (hGH) signal transduction initiates with a receptor dimerization in which one molecule binds to the receptor through sites 1 and 2. A sandwich enzyme-linked immunosorbent assay was developed for quantifying hGH molecules that present helix 4 from binding site 1. For this, horse anti-rhGH antibodies were eluted by an immunoaffinity column constituted by sepharose-rhGH. These antibodies were purified through a second column with synthetic peptide correspondent to hGH helix 4 , immobilized to sepharose, and used as capture antibodies. Those that did not recognize synthetic peptide were used as a marker antibody. The working range was of 1.95 to 31.25 ng/mL of hGH. The intra-assay coefficient of variation (CV) was between 4.53% and 6.33%, while the interassay CV was between 6.00% and 8.27%. The recovery range was between 96.0% to 103.8%. There was no cross-reactivity with human prolactin. These features show that our assay is an efficient method for the determination of hGH.
Related collections
Most cited references29
- Record: found
- Abstract: found
- Article: not found
A hot spot of binding energy in a hormone-receptor interface.
- Record: found
- Abstract: found
- Article: not found
High-resolution epitope mapping of hGH-receptor interactions by alanine-scanning mutagenesis.
- Record: found
- Abstract: not found
- Article: not found
