- Record: found
- Abstract: found
- Article: not found
Cost-Effectiveness of Rivaroxaban Versus Warfarin for Stroke Prevention in Atrial Fibrillation in the Belgian Healthcare Setting
Read this article at
Abstract
Background
Warfarin, an inexpensive drug that has been available for over half a century, has been the mainstay of anticoagulant therapy for stroke prevention in patients with atrial fibrillation (AF). Recently, rivaroxaban, a novel oral anticoagulant (NOAC) which offers some distinct advantages over warfarin, the standard of care in a world without NOACs, has been introduced and is now recommended by international guidelines.
Objective
The aim of this study was to evaluate, from a Belgian healthcare payer perspective, the cost-effectiveness of rivaroxaban versus use of warfarin for the treatment of patients with non-valvular AF at moderate to high risk.
Methods
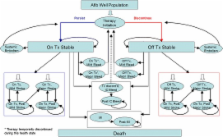
A Markov model was designed and populated with local cost estimates, safety-on-treatment clinical results from the pivotal phase III ROCKET AF trial and utility values obtained from the literature.
Results
Rivaroxaban treatment was associated with fewer ischemic strokes and systemic embolisms (0.308 vs. 0.321 events), intracranial bleeds (0.048 vs. 0.063), and myocardial infarctions (0.082 vs. 0.095) per patient compared with warfarin. Over a lifetime time horizon, rivaroxaban led to a reduction of 0.042 life-threatening events per patient, and increases of 0.111 life-years and 0.094 quality-adjusted life-years (QALYs) versus warfarin treatment. This resulted in an incremental cost-effectiveness ratio of €8,809 per QALY or €7,493 per life-year gained. These results are based on valuated data from 2010. Sensitivity analysis indicated that these results were robust and that rivaroxaban is cost-effective compared with warfarin in 87 % of cases should a willingness-to-pay threshold of €35,000/QALY gained be considered.
Related collections
Most cited references19
- Record: found
- Abstract: found
- Book: not found
Decision Modelling for Health Economic Evaluation
- Record: found
- Abstract: found
- Article: not found
Effect of intensity of oral anticoagulation on stroke severity and mortality in atrial fibrillation.
- Record: found
- Abstract: found
- Article: not found
