- Record: found
- Abstract: found
- Article: found
Immune Checkpoint Inhibitor Induced Pericarditis and Encephalitis in a Patient Treated With Ipilimumab and Nivolumab for Metastatic Melanoma: A Case Report and Review of the Literature
Read this article at
Abstract
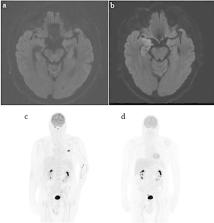
Immune checkpoint inhibitors (ICIs) have dramatically improved outcomes in melanoma. Common ICI toxicities have become familiar to clinicians; however, rare delayed toxicities remain challenging given the paucity of data with such presentations. We present the unique case of a 61-year-old with metastatic melanoma with two rare, delayed ICI-induced toxicities. After resection of a large symptomatic parietal metastases, this patient received two doses of combination ipilimumab and nivolumab. Five weeks following his second dose, he developed ICI-induced pericarditis with associated pericardial effusion and early signs of tamponade. Corticosteroids were not administered due to a concurrent cerebral abscess. Administration of colchicine, ibuprofen, judicious monitoring, and cessation of immunotherapy led to the complete resolution of the effusion over several weeks. Seven months following his last dose of immunotherapy, the patient developed ICI-associated grade four autoimmune encephalitis, presenting as status epilepticus. High-dose steroid initiation led to rapid clinical improvement. The patient remains in near-complete response on imaging with no recurrence of pericardial effusion and partial resolution of neurological symptoms. ICI-induced pericardial disease and encephalitis carry substantial mortality rates and prompt diagnosis and management is critical. Clinicians must therefore remain vigilant for these rare toxicities regardless of duration of drug exposure or time since cessation of therapy.
Related collections
Most cited references13
- Record: found
- Abstract: not found
- Article: not found
Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma
- Record: found
- Abstract: found
- Article: not found
Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors
- Record: found
- Abstract: found
- Article: not found