- Record: found
- Abstract: found
- Article: found
Ionic and cellular mechanisms underlying TBX5/PITX2 insufficiency-induced atrial fibrillation: Insights from mathematical models of human atrial cells
Read this article at
Abstract
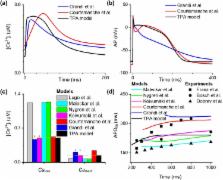
Transcription factors TBX5 and PITX2 involve in the regulation of gene expression of ion channels and are closely associated with atrial fibrillation (AF), the most common cardiac arrhythmia in developed countries. The exact cellular and molecular mechanisms underlying the increased susceptibility to AF in patients with TBX5/PITX2 insufficiency remain unclear. In this study, we have developed and validated a novel human left atrial cellular model (TPA) based on the ten Tusscher-Panfilov ventricular cell model to systematically investigate how electrical remodeling induced by TBX5/PITX2 insufficiency leads to AF. Using our TPA model, we have demonstrated that spontaneous diastolic depolarization observed in atrial myocytes with TBX5-deletion can be explained by altered intracellular calcium handling and suppression of inward-rectifier potassium current ( I K1 ). Additionally, our computer simulation results shed new light on the novel cellular mechanism underlying AF by indicating that the imbalance between suppressed outward current I K1 and increased inward sodium-calcium exchanger current ( I NCX ) resulted from SR calcium leak leads to spontaneous depolarizations. Furthermore, our simulation results suggest that these arrhythmogenic triggers can be potentially suppressed by inhibiting sarcoplasmic reticulum (SR) calcium leak and reversing remodeled I K1 . More importantly, this study has clinically significant implications on the drugs used for maintaining SR calcium homeostasis, whereby drugs such as dantrolene may confer significant improvement for the treatment of AF patients with TBX5/PITX2 insufficiency.
Related collections
Most cited references57
- Record: found
- Abstract: found
- Article: not found
GATA4 mutations cause human congenital heart defects and reveal an interaction with TBX5.
- Record: found
- Abstract: found
- Article: not found
Variants conferring risk of atrial fibrillation on chromosome 4q25.
- Record: found
- Abstract: found
- Article: not found