- Record: found
- Abstract: found
- Article: found
PLK1 regulates hepatic stellate cell activation and liver fibrosis through Wnt/β‐catenin signalling pathway
Read this article at
Abstract
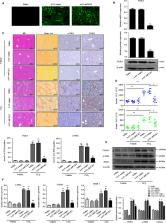
As an outcome of chronic liver disease, liver fibrosis involves the activation of hepatic stellate cells (HSCs) caused by a variety of chronic liver injuries. It is important to explore approaches to inhibit the activation and proliferation of HSCs for the treatment of liver fibrosis. PLK1 is overexpressed in many human tumour cells and has become a popular drug target in tumour therapy. Therefore, further study of the function of PLK1 in the cell cycle is valid. In the present study, we found that PLK1 expression was elevated in primary HSCs isolated from CCl 4‐induced liver fibrosis mice and LX‐2 cells stimulated with TGF‐β1. Knockdown of PLK1 inhibited α‐SMA and Col1α1 expression and reduced the activation of HSCs in CCl 4‐induced liver fibrosis mice and LX‐2 cells stimulated with TGF‐β1. We further showed that inhibiting the expression of PLK1 reduced the proliferation of HSCs and promoted HSCs apoptosis in vivo and in vitro. Furthermore, we found that the Wnt/β‐catenin signalling pathway may be essential for PLK1‐mediated HSCs activation. Together, blocking PLK1 effectively suppressed liver fibrosis by inhibiting HSC activation, which may provide a new treatment strategy for liver fibrosis.
Related collections
Most cited references27
- Record: found
- Abstract: not found
- Article: not found
LECT2, a Ligand for Tie1, Plays a Crucial Role in Liver Fibrogenesis
- Record: found
- Abstract: found
- Article: not found
Tyrosine kinase SYK is a potential therapeutic target for liver fibrosis

- Record: found
- Abstract: found
- Article: found