- Record: found
- Abstract: found
- Article: found
Cost-effectiveness analysis of anlotinib versus sunitinib as first-line treatment for metastatic renal cell carcinoma in China
Read this article at
Abstract
Background
Sunitinib was approved several years ago as a first-line drug for treating metastatic renal cell carcinoma (mRCC); however, its high price and broad side effects when administered at the standard dose have limited its clinical use. A clinical trial (NCT02072031) confirmed that anlotinib could be used as the first-line treatment for mRCC. This study was conducted to evaluate the cost-effectiveness of anlotinib as a first-line treatment for mRCC compared to that of sunitinib in China.
Methods
A Markov model was established to compare the cost-effectiveness of anlotinib with that of sunitinib. Clinical data were obtained from a multi-center phase II trial (clinical trial information: NCT02072031). Utility values were obtained from the literature. Total costs were calculated from a Chinese societal perspective. A sensitivity analysis was conducted to assess the model uncertainty. The life-year (LY), quality-adjusted life-year (QALY), and incremental cost-effectiveness ratio were calculated.
Results
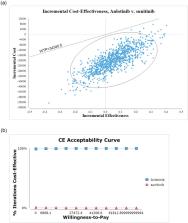
The base-case analysis over a lifetime horizon of 10 years showed that the anlotinib group had 2.196 LYs and 1.487 QALYs at a total cost of $68,597.84. The sunitinib group had 2.194 LYs and 1.432 QALYs at a total cost of $88,060.02. This resulted in incremental cost-effectiveness ratios (ICER) of anlotinib versus sunitinib of $-9,210,858.93 per LYs and $-354,117.07 per QALYs, suggesting that anlotinib is a more effective and less costly strategy than sunitinib.
Related collections
Most cited references20
- Record: found
- Abstract: found
- Article: not found
Pazopanib versus sunitinib in metastatic renal-cell carcinoma.
- Record: found
- Abstract: not found
- Article: not found