- Record: found
- Abstract: found
- Article: found
Soft wireless sternal patch to detect systemic vasoconstriction using photoplethysmography
Read this article at
Summary
Vasoconstriction is a crucial physiological process that serves as the body’s primary blood pressure regulation mechanism and a key marker of numerous harmful health conditions. The ability to detect vasoconstriction in real time would be crucial for detecting blood pressure, identifying sympathetic arousals, characterizing patient wellbeing, detecting sickle cell anemia attacks early, and identifying complications caused by hypertension medications. However, vasoconstriction manifests weakly in traditional photoplethysmogram (PPG) measurement locations, like the finger, toe, and ear. Here, we report a wireless, fully integrated, soft sternal patch to capture PPG signals from the sternum, an anatomical region that exhibits a robust vasoconstrictive response. With healthy controls, the device is highly capable of detecting vasoconstriction induced endogenously and exogenously. Furthermore, in overnight trials with patients with sleep apnea, the device shows a high agreement (r 2 = 0.74) in vasoconstriction detection with a commercial system, demonstrating its potential use in portable, continuous, long-term vasoconstriction monitoring.
Graphical abstract
Highlights
-
•
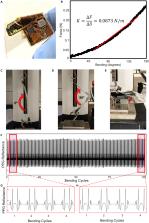
Optimized skin-conformality enables continuous PPG monitoring from the sternum
-
•
Improvements in sensor mechanics reduce motion artifacts compared to rigid devices
-
•
We demonstrate high-agreement (78%) vasoconstrictions compared to WatchPAT
-
•
Vasoconstriction monitoring could play a crucial role in monitoring cardiac disease
Abstract
Health sciences; Biological sciences; Biotechnology; Bioelectronics; Engineering
Related collections
Most cited references29
- Record: found
- Abstract: found
- Article: not found
Respiration signals from photoplethysmography.
- Record: found
- Abstract: not found
- Article: not found
The α1D-adrenergic receptor directly regulates arterial blood pressure via vasoconstriction

- Record: found
- Abstract: found
- Article: found
