- Record: found
- Abstract: found
- Article: found
Aβ oligomer concentration in mouse and human brain and its drug-induced reduction ex vivo
Read this article at
Summary
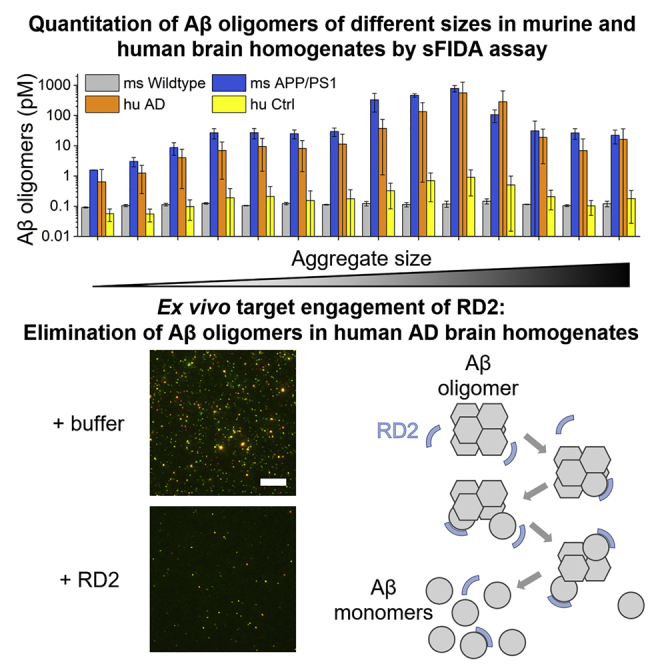
The elimination of amyloid beta (Aβ) oligomers is a promising strategy for therapeutic drug development of Alzheimer’s disease (AD). AD mouse models that develop Aβ pathology have been used to demonstrate in vivo efficacy of compounds that later failed in clinical development. Here, we analyze the concentration and size distribution of Aβ oligomers in different transgenic mouse models of AD and in human brain samples by surface-based fluorescence intensity distribution analysis (sFIDA), a highly sensitive method for detecting and quantitating protein aggregates. We demonstrate dose- and time-dependent oligomer elimination by the compound RD2 in mouse and human AD brain homogenates as sources of native Aβ oligomers. Such ex vivo target engagement analyses with mouse- and human-brain-derived oligomers have the potential to enhance the translational value from pre-clinical proof-of-concept studies to clinical trials.
Graphical abstract
Highlights
Abstract
Eliminating amyloid beta (Aβ) oligomers is a promising strategy for therapeutic drug development of Alzheimer’s disease (AD). Here, Kass et al. quantitate Aβ oligomers in brain homogenates from various AD murine and human tissue samples and demonstrate the dose- and time-dependent disassembly of Aβ oligomers by the compound RD2.
Related collections
Most cited references64
- Record: found
- Abstract: found
- Article: not found
Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease.
- Record: found
- Abstract: found
- Article: not found