- Record: found
- Abstract: found
- Article: found
In vivo study of doxorubicin-loaded cell-penetrating peptide-modified pH-sensitive liposomes: biocompatibility, bio-distribution, and pharmacodynamics in BALB/c nude mice bearing human breast tumors
Abstract
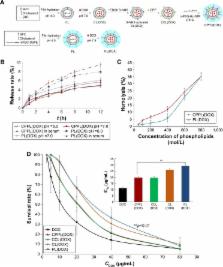
In vivo evaluation of drug delivery vectors is essential for clinical translation. In BALB/c nude mice bearing human breast cancer tumors, we investigated the biocompatibility, pharmacokinetics, and pharmacodynamics of doxorubicin (DOX)-loaded novel cell-penetrating peptide (CPP)-modified pH-sensitive liposomes (CPPL) (referred to as CPPL(DOX)) with an optimal CPP density of 4%. In CPPL, a polyethylene glycol (PEG) derivative formed by conjugating PEG with stearate via acid-degradable hydrazone bond (PEG2000-Hz-stearate) was inserted into the surface of liposomes, and CPP was directly attached to liposome surfaces via coupling with stearate to simultaneously achieve long circulation time in blood and improve the selectivity and efficacy of CPP for tumor targeting. Compared to PEGylated liposomes, CPPL enhanced DOX accumulation in tumors up to 1.9-fold ( p<0.01) and resulted in more cell apoptosis as a result of DNA disruption as well as a relatively lower tumor growth ratio (T/C%). Histological examination did not show any signs of necrosis or inflammation in normal tissues, but large cell dissolving areas were found in tumors following the treatment of animals with CPPL(DOX). Our findings provide important and detailed information regarding the distribution of CPPL(DOX) in vivo and reveal their abilities of tumor penetration and potential for the treatment of breast cancer.
Most cited references33
- Record: found
- Abstract: found
- Article: not found
Delivery of macromolecules using arginine-rich cell-penetrating peptides: ways to overcome endosomal entrapment.
- Record: found
- Abstract: found
- Article: not found
Accelerated blood clearance (ABC) phenomenon upon repeated injection of PEGylated liposomes.
- Record: found
- Abstract: found
- Article: not found