- Record: found
- Abstract: found
- Article: found
Preference for a prefilled syringe or an auto-injection device for delivering golimumab in patients with moderate-to-severe ulcerative colitis: a randomized crossover study
Abstract
Purpose
Simponi ® (golimumab, MSD) is a fully human monoclonal antibody against tumor necrosis factor alpha administered subcutaneously using an autoinjector or a prefilled syringe. This study examined preference for administration of golimumab by autoinjector or prefilled syringe in patients with moderate-to-severe ulcerative colitis (UC).
Patients and methods
This was a multicenter, open-label, randomized crossover trial (EudraCT no 2014-000656-29). Patients with moderate-to-severe UC were randomized 1:1 to receive 2 subcutaneous injections of 50 mg golimumab with the autoinjector followed by 2 injections of 50 mg with the prefilled syringe or the same 4 injections administered in the opposite order. Patients assessed preference, ease of use, and discomfort immediately after the injections and 2 weeks later.
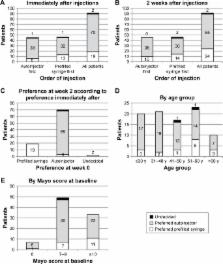
Results
Ninety-one patients were included (median age=42.7 years [range, 19.7–93.7]; 58% male). The autoinjector was preferred by 76.9% of patients immediately after injections and by 71.4% 2 weeks later. The autoinjector was more often considered extremely easy or easy to use (94.5%) than the prefilled syringe (73.6%). Moderate discomfort or worse was reported by more patients when using the prefilled syringe (20.9%) than when using the autoinjector (5.5%), and severe discomfort or discomfort preventing injection of future doses was reported by 8.8% for the pre-filled syringe but not at all when using the autoinjector. A favorable or extremely favorable overall impression was reported by 89.0% for the autoinjector and 72.5% for the prefilled syringe.
Most cited references19
- Record: found
- Abstract: not found
- Article: not found
European evidence-based Consensus on the diagnosis and management of ulcerative colitis: Definitions and diagnosis.
- Record: found
- Abstract: found
- Article: not found