- Record: found
- Abstract: found
- Article: found
Discontinuation of Dapoxetine Treatment in Patients With Premature Ejaculation: A 2-Year Prospective Observational Study
Read this article at
Abstract
Introduction
Although dapoxetine is the only oral pharmacologic agent approved for the treatment of premature ejaculation (PE) and is very effective, the discontinuation rate is high.
Aim
To assess the discontinuation rate of patients with PE and the reasons for discontinuation in real-world practice.
Methods
In total, 182 consecutive patients were enrolled. Type of PE, self-estimated intravaginal ejaculation latency time, and medical history were evaluated in all patients who also completed the erectile function domain of the International Index of Erectile Function (IIEF). Visits were scheduled 1, 3, 6, 12, and 24 months after initiation of therapy; treatment status and the reasons for discontinuation in those who did discontinue were checked. The relations of discontinuation rates were compared with various parameters and the time to discontinuation after treatment commencement.
Results
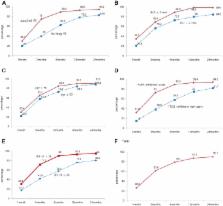
Of all patients, 9.9% continued treatment to 2 years. The cumulative discontinuation rates at 1, 3, 6, 12, and 24 months were 26.4%, 61.6%, 79.1%, 87.3%, and 90.1%, respectively. Moreover, 79.1% of all patients discontinued treatment within 6 months. After 12 months, the discontinuation rate decreased sharply. The reasons for discontinuation were cost (29.9%), disappointment that PE was not curable and that dapoxetine was required every time sexual intercourse was contemplated (25%), side effects (11.6%), perceived poor efficacy (9.8%), a search for other treatment options (5.5%), and unknown (18.3%). Patients with acquired PE (vs lifelong PE), with intravaginal ejaculation latency time longer than 2 minutes before treatment, on phosphodiesterase type 5 inhibitors, and with IIEF erectile function scores lower than 26 tended to discontinue early and thus exhibited high dropout rates.
Conclusion
The treatment discontinuation rate of dapoxetine was very high. The main reasons for discontinuation were the cost and disappointment that treatment was required every time adequate sexual function was required.
Park HJ, Park NC, Kim TN, et al. Discontinuation of Dapoxetine Treatment in Patients With Premature Ejaculation: A 2-Year Prospective Observational Study. Sex Med 2017;5:e99–e105.
Related collections
Most cited references29

- Record: found
- Abstract: found
- Article: found
An Update of the International Society of Sexual Medicine's Guidelines for the Diagnosis and Treatment of Premature Ejaculation (PE)
- Record: found
- Abstract: found
- Article: not found
Efficacy and tolerability of dapoxetine in treatment of premature ejaculation: an integrated analysis of two double-blind, randomised controlled trials.
- Record: found
- Abstract: found
- Article: not found