- Record: found
- Abstract: found
- Article: found
Modulation of cue-induced firing of ventral tegmental area dopamine neurons by leptin and ghrelin
Read this article at
Abstract
Background/objectives:
The rewarding value of palatable foods contributes to overconsumption, even in satiated subjects. Midbrain dopaminergic activity in response to reward-predicting environmental stimuli drives reward-seeking and motivated behavior for food rewards. This mesolimbic dopamine (DA) system is sensitive to changes in energy balance, yet it has thus far not been established whether reward signaling of DA neurons in vivo is under control of hormones that signal appetite and energy balance such as ghrelin and leptin.
Subjects/methods:
We trained rats ( n=11) on an operant task in which they could earn two different food rewards. We then implanted recording electrodes in the ventral tegmental area (VTA), and recorded from DA neurons during behavior. Subsequently, we assessed the effects of mild food restriction and pretreatment with the adipose tissue-derived anorexigenic hormone leptin or the orexigenic hormone ghrelin on VTA DA reward signaling.
Results:
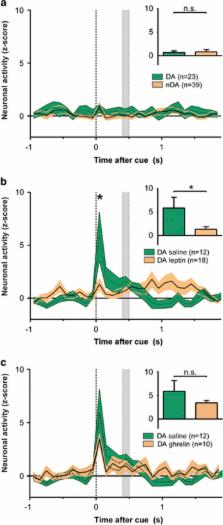
Animals showed an increase in performance following mild food restriction ( P=0.002). Importantly, food-cue induced DA firing increased when animals were food restricted ( P=0.02), but was significantly attenuated after leptin pretreatment ( P=0.00). While ghrelin did affect baseline DA activity ( P=0.025), it did not affect cue-induced firing ( P⩾0.353).
Conclusions:
Metabolic signals, such as leptin, affect food seeking, a process that is dependent on the formation of cue-reward outcomes and involves midbrain DA signaling. These data show that food restriction engages the encoding of food cues by VTA DA neurons at a millisecond level and leptin suppresses this activity. This suggests that leptin is a key in linking metabolic information to reward signaling.
Related collections
Most cited references36
- Record: found
- Abstract: found
- Article: not found
Leptin receptor signaling in midbrain dopamine neurons regulates feeding.
- Record: found
- Abstract: found
- Article: not found
The control of firing pattern in nigral dopamine neurons: burst firing.
- Record: found
- Abstract: found
- Article: not found