- Record: found
- Abstract: found
- Article: found
High serum levels of Dickkopf-1 are associated with a poor prognosis in prostate cancer patients
Read this article at
Abstract
Background
The Wnt inhibitor Dickkopf-1 (DKK-1) has been linked to the progression of malignant bone disease by impairing osteoblast activity. In addition, there is increasing data to suggest direct tumor promoting effects of DKK-1. The prognostic role of DKK-1 expression in prostate cancer remains unclear.
Methods
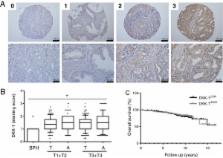
A prostate cancer tissue microarray (n = 400) was stained for DKK-1 and DKK-1 serum levels were measured in 80 patients with prostate cancer. The independent prognostic value of DKK-1 expression was assessed using multivariate analyses.
Results
DKK-1 tissue expression was significantly increased in prostate cancer compared to benign disease, but was not correlated with survival. However, high DKK-1 serum levels at the time of the diagnosis were associated with a significantly shorter overall and disease-specific survival. Multivariate analyses defined high serum levels of DKK-1 as an independent prognostic marker in prostate cancer (HR 3.73; 95%CI 1.44-9.66, p = 0.007).
Conclusion
High DKK-1 serum levels are associated with a poor survival in patients with prostate cancer. In light of current clinical trials evaluating the efficacy of anti-DKK-1 antibody therapies in multiple myeloma and solid malignancies, the measurement of DKK-1 in prostate cancer may gain clinical relevance.
Related collections
Most cited references13
- Record: found
- Abstract: found
- Article: not found
Anti-DKK1 mAb (BHQ880) as a potential therapeutic agent for multiple myeloma.
- Record: found
- Abstract: found
- Article: not found
Increased Dickkopf-1 expression in breast cancer bone metastases
- Record: found
- Abstract: found
- Article: not found