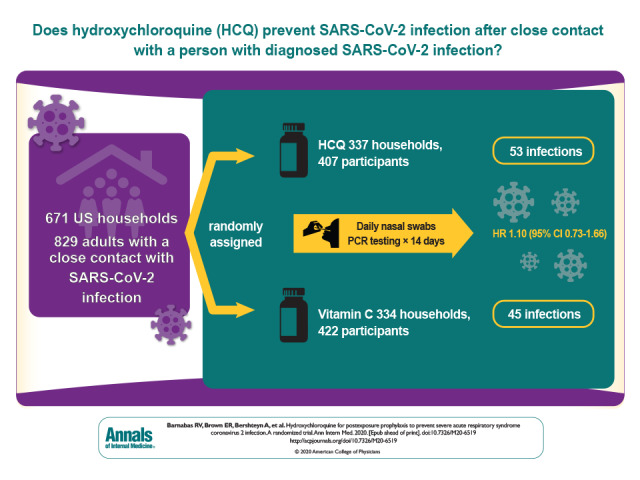
Acknowledgment: The authors thank the study participants for their motivation and dedication; the
members of the trial's Data and Safety Monitoring Board (Drs. David Glidden, Michael
Boeckh, and Robert Coombs), local advisors at each trial site, and overseeing ethics
review committees for their expertise and guidance; Drs. Scott Miller and Peter Dull
from the Bill & Melinda Gates Foundation for their attentive oversight; and the HCQ
COVID-19 PEP Study Team for their dedication and perseverance.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily
represent the views, decisions, or policies of the institutions with which they are
affiliated or the HCQ COVID-19 PEP Study funders.
Funding: The HCQ COVID-19 PEP Study was funded by the Bill & Melinda Gates Foundation (INV-016204)
through the COVID-19 Therapeutics Accelerator and the University of Washington King
K. Holmes Endowed Professorship in STDs and AIDS. Hydroxychloroquine for the study
was donated by Sandoz.
Reproducible Research Statement:
Study protocol: Available at ClinicalTrials.gov (NCT02929992).
Statistical code: Statistical code: The Cox model fit call is provided in
Supplement
Table 1; other file management aspects of the statistical code are not available.
Data set: A complete deidentified patient data set sufficient to reproduce the study findings
will be made available no later than 1 year after completion of the trial, after approval
of a concept sheet summarizing the analyses to be done. Further inquiries can be directed
to the HCQ COVID-19 PEP Study Scientific Committee (e-mail,
icrc@
123456uw.edu
).
Corresponding Author: Ruanne V. Barnabas, International Clinical Research Center (ICRC), Department of
Global Health, University of Washington, UW Box 359927, 325 Ninth Avenue, Seattle,
WA 98104; e-mail,
rbarnaba@
123456uw.edu
.
Current Author Addresses: Drs. Barnabas, Stankiewicz Karita, Morrison, Celum, and Baeten; Mr. Schaafsma; Ms.
Krows; Ms. Thomas; Mr. Haugen; and Ms. Kidoguchi: International Clinical Research
Center (ICRC), Department of Global Health, University of Washington, UW Box 359927,
325 Ninth Avenue, Seattle, WA 98104.
Dr. Brown: Fred Hutchinson Cancer Research Center, 1100 Fairview Avenue North, Seattle,
WA 98109.
Dr. Bershteyn: Translational Research Building, 227 East 30th Street, New York, NY
10016.
Dr. Johnston: International Clinical Research Center (ICRC), Department of Global
Health, University of Washington, UW Box 359928, 325 Ninth Avenue, Seattle, WA 98104.
Dr. Thorpe: NYU Grossman School of Medicine, 180 Madison Avenue, New York, NY 10016.
Dr. Kottkamp: Bellevue Hospital, 462 First Avenue, H Building, 16S 5-13, New York,
NY 10016.
Drs. Neuzil, Laufer, and Deming: Center for Vaccine Development and Global Health,
University of Maryland School of Medicine, 685 West Baltimore Street, Room 480, Baltimore,
MD 21201.
Dr. Paasche-Orlow: Boston Medical Center, 801 Massachusetts Avenue, Second Floor,
Boston, MA 02119.
Dr. Kissinger: Tulane University School of Public Health and Tropical Medicine, 1440
Canal Street, Suite 2004, New Orleans, LA 70112.
Dr. Luk: Tulane University Health Sciences Center, Section of Infectious Disease,
1415 Tulane Avenue, New Orleans, LA 70112.
Dr. Paolino: Upstate Medical University, Infectious Disease Division, 725 Irving Avenue,
Suite 311, Syracuse, NY 13210.
Dr. Landovitz: UCLA Center for Clinical AIDS Research & Education, 911 Broxton Avenue,
Suite 200, Los Angeles, CA 90024.
Dr. Hoffman: UCLA Department of Medicine, Division of Infectious Diseases, 10833 Le
Conte Avenue, 52-215 CHS, Los Angeles, CA 90095.
Dr. Wener: University of Washington Medical Center, Department of Laboratory Medicine
and Pathology, Box 357110, Seattle, WA 98195.
Drs. Greninger and Huang: University of Washington, Virology Laboratory, 1616 Eastlake
Avenue East, Suite 320, Seattle, WA 98102.
Dr. Jerome: Fred Hutchinson Cancer Research Center, 1100 Fairview Avenue North, E5-110,
Seattle, WA 98109.
Dr. Wald: University of Washington, 325 Ninth Avenue, HMC#359928, Seattle, WA 98104.
Dr. Chu: University of Washington, 750 Republican Street, UWMC#358061, Seattle, WA
98109.
Author Contributions: Conception and design: R.V. Barnabas, E.R. Brown, A. Bershteyn, K.M. Neuzil, M.L.
Krows, M. Wener, A. Wald, C. Celum, H.Y. Chu, J.M. Baeten.
Analysis and interpretation of the data: R.V. Barnabas, E.R. Brown, C. Johnston, L.E.
Thorpe, K.M. Neuzil, M.K. Laufer, P.J. Kissinger, K. Paolino, R.J. Landovitz, R.M.
Hoffman, T.T. Schaafsma, K.K. Thomas, L. Kidoguchi, M.L. Huang, K.R. Jerome, A. Wald,
C. Celum, H.Y. Chu, J.M. Baeten.
Drafting of the article: R.V. Barnabas, E.R. Brown, K.M. Neuzil, M.K. Laufer, P.J.
Kissinger, T.T. Schaafsma, M.L. Krows, C. Celum.
Critical revision for important intellectual content: R.V. Barnabas, E.R. Brown, A.
Bershteyn, H.C. Stankiewicz Karita, C. Johnston, L.E. Thorpe, A. Kottkamp, K.M. Neuzil,
M.K. Laufer, M.K. Paasche-Orlow, P.J. Kissinger, K. Paolino, R.J. Landovitz, R.M.
Hoffman, T.T. Schaafsma, K.K. Thomas, S. Morrison, M. Wener, A. Wald, H.Y. Chu, J.M.
Baeten.
Final approval of the article: R.V. Barnabas, E.R. Brown, A. Bershteyn, H.C. Stankiewicz
Karita, C. Johnston, L.E. Thorpe, A. Kottkamp, K.M. Neuzil, M.K. Laufer, M. Deming,
M.K. Paasche-Orlow, P.J. Kissinger, A. Luk, R.J. Landovitz, K. Paolino, R.M. Hoffman,
T.T. Schaafsma, M.L. Krows, K.K. Thomas, S. Morrison, H.S. Haugen, L. Kidoguchi, M.
Wener, A.L. Greninger, M.L. Huang, K.R. Jerome, A. Wald, C. Celum, H.Y. Chu, J.M.
Baeten.
Provision of study materials or patients: H.C. Stankiewicz Karita, A. Kottkamp, M.K.
Laufer, M. Deming, M.K. Paasche-Orlow, P.J. Kissinger, A. Luk, R.J. Landovitz, R.M.
Hoffman, A. Wald.
Statistical expertise: E.R. Brown, T.T. Schaafsma, K.K. Thomas.
Obtaining of funding: R.V. Barnabas, J.M. Baeten.
Administrative, technical, or logistic support: H.C. Stankiewicz Karita, L.E. Thorpe,
A. Kottkamp, M.K. Laufer, M.K. Paasche-Orlow, A.L. Greninger, H.S. Haugen, M. Wener,
M.L. Huang, K.R. Jerome, A. Wald, J.M. Baeten.
Collection and assembly of data: A. Bershteyn, H.C. Stankiewicz Karita, C. Johnston,
L.E. Thorpe, A Kottkamp, K.M. Neuzil, M.K. Laufer, M.K. Paasche-Orlow, P.J. Kissinger,
K. Paolino, R.J. Landovitz, R.M. Hoffman, T.T. Schaafsma, S. Morrison, A.L. Greninger,
M.L. Huang, K.R. Jerome, C. Celum, J.M. Baeten.