- Record: found
- Abstract: found
- Article: found
Placebo‐Controlled Randomized Trial of an Intestinal Bile Salt Transport Inhibitor for Pruritus in Alagille Syndrome
Read this article at
Abstract
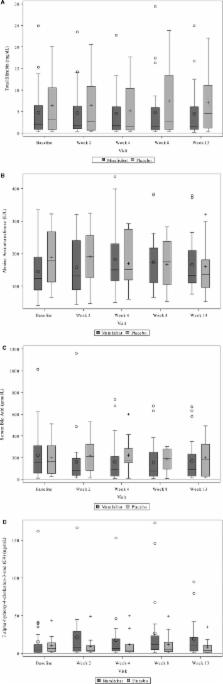
Medically refractory, severe, cholestasis‐induced pruritus in Alagille syndrome may be improved by surgical interruption of the enterohepatic circulation. This multicenter trial (NCT02057692) tested the hypothesis that the intestinal bile acid transport inhibitor maralixibat would similarly reduce pruritus in Alagille syndrome. Thirty‐seven children with Alagille syndrome were randomly assigned to double‐blinded administration of placebo, 70, 140, or 280 µg/kg/day of maralixibat for 13 weeks. Pruritus was assessed by caregiver (itch‐reported outcome instrument [ItchRO]) and clinician report (range, 0‐4 [severe]). Liver chemistries and serum bile acids were measured. The primary outcome was the change from baseline to week 13 in ItchRO relative to placebo. In the a priori first analysis of the primary efficacy endpoint, the mean adjusted difference between participants receiving 140 or 280 µg/kg/day and placebo was –0.47 (95% confidence interval [CI], –1.14, 0.20; P = 0.16). Statistically significant decreases were observed with doses of 70 and 140 µg/kg/day (mean adjusted difference, –0.89; 95% CI, –1.70, –0.08; P = 0.032; and mean adjusted difference, –0.91; 95% CI, –1.62, –0.19; P = 0.014) but not 280 µg/kg/day (mean adjusted difference, –0.04; 95% CI, –0.94, 0.86; P = 0.44) or all doses combined (mean adjusted difference, –0.61; 95% CI, –1.24, 0.20; P = 0.055). A 1‐point reduction in pruritus was more common in maralixibat‐treated versus placebo‐treated participants (caregiver ItchRO, 65% versus 25%; P = 0.06; clinician score, 76% versus 25%; P = 0.01). There were no significant changes in liver chemistries or bile acids relative to placebo. Adverse and serious adverse events were similar between maralixibat and placebo. Conclusion: Although the prespecified primary analyses of ItchRO were not all statistically significant, the data suggest that maralixibat is safe and may reduce pruritus in Alagille syndrome.
Related collections
Most cited references16
- Record: found
- Abstract: found
- Article: not found
Effect of partial ileal bypass surgery on mortality and morbidity from coronary heart disease in patients with hypercholesterolemia. Report of the Program on the Surgical Control of the Hyperlipidemias (POSCH)
- Record: found
- Abstract: not found
- Article: not found
Effect of ileal bile acid transporter inhibitor GSK2330672 on pruritus in primary biliary cholangitis: a double-blind, randomised, placebo-controlled, crossover, phase 2a study
- Record: found
- Abstract: found
- Article: not found