- Record: found
- Abstract: found
- Article: found
Nrf2-Keap1 pathway promotes cell proliferation and diminishes ferroptosis
Read this article at
Abstract
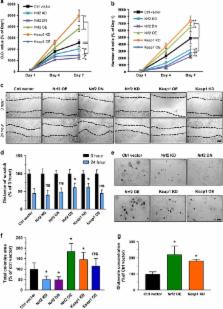
Cancer cells are hallmarked by high proliferation and imbalanced redox consumption and signaling. Various oncogenic pathways such as proliferation and evading cell death converge on redox-dependent signaling processes. Nrf2 is a key regulator in these redox-dependent events and operates in cytoprotection, drug metabolism and malignant progression in cancer cells. Here, we show that patients with primary malignant brain tumors (glioblastomas, WHO °IV gliomas, GBM) have a devastating outcome and overall reduced survival when Nrf2 levels are upregulated. Nrf2 overexpression or Keap1 knockdown in glioma cells accelerate proliferation and oncogenic transformation. Further, activation of the Nrf2-Keap1 signaling upregulates xCT (aka SLC7A11 or system X c −) and amplifies glutamate secretion thereby impacting on the tumor microenvironment. Moreover, both fostered Nrf2 expression and conversely Keap1 inhibition promote resistance to ferroptosis. Altogether, the Nrf2-Keap1 pathway operates as a switch for malignancy in gliomas promoting cell proliferation and resistance to cell death processes such as ferroptosis. Our data demonstrate that the Nrf2-Keap1 pathway is critical for cancer cell growth and operates on xCT. Nrf2 presents the Achilles’ heel of cancer cells and thus provides a valid therapeutic target for sensitizing cancer for chemotherapeutics.
Related collections
Most cited references37
- Record: found
- Abstract: found
- Article: not found
Guidelines for the selection of highly effective siRNA sequences for mammalian and chick RNA interference.
- Record: found
- Abstract: found
- Article: not found