- Record: found
- Abstract: found
- Article: found
Targeting anandamide metabolism rescues core and associated autistic-like symptoms in rats prenatally exposed to valproic acid
Read this article at
Abstract
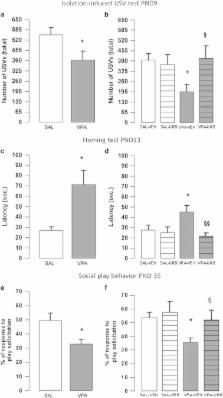
Autism spectrum disorders (ASD) are characterized by altered sociability, compromised communication and stereotyped/repetitive behaviors, for which no specific treatments are currently available. Prenatal exposure to valproic acid (VPA) is a known, although still underestimated, environmental risk factor for ASD. Altered endocannabinoid activity has been observed in autistic patients, and endocannabinoids are known to modulate behavioral traits that are typically affected in ASD. On this basis, we tested the hypothesis that changes in the endocannabinoid tone contribute to the altered phenotype induced by prenatal VPA exposure in rats, with focus on behavioral features that resemble the core and associated symptoms of ASD. In the course of development, VPA-exposed rats showed early deficits in social communication and discrimination, compromised sociability and social play behavior, stereotypies and increased anxiety, thus providing preclinical proof of the long-lasting deleterious effects induced by prenatal VPA exposure. At the neurochemical level, VPA-exposed rats displayed altered phosphorylation of CB1 cannabinoid receptors in different brain areas, associated with changes in anandamide metabolism from infancy to adulthood. Interestingly, enhancing anandamide signaling through inhibition of its degradation rescued the behavioral deficits displayed by VPA-exposed rats at infancy, adolescence and adulthood. This study therefore shows that abnormalities in anandamide activity may underlie the deleterious impact of environmental risk factors on ASD-relevant behaviors and that the endocannabinoid system may represent a therapeutic target for the core and associated symptoms displayed by autistic patients.
Related collections
Most cited references71
- Record: found
- Abstract: not found
- Article: not found
The molecular logic of endocannabinoid signalling.
- Record: found
- Abstract: found
- Article: not found
Role of endogenous cannabinoids in synaptic signaling.
- Record: found
- Abstract: found
- Article: not found