- Record: found
- Abstract: found
- Article: found
Hydrogen sulfide ameliorates chronic renal failure in rats by inhibiting apoptosis and inflammation through ROS/MAPK and NF- κB signaling pathways
Read this article at
Abstract
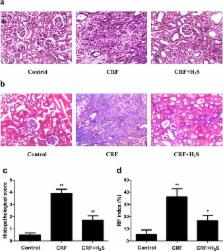
Chronic renal failure (CRF) is a major public health problem worldwide. Hydrogen sulfide (H 2S) plays important roles in renal physiological and pathophysiological processes. However, whether H 2S could protect against CRF in rats remains unclear. In this study, we found that H 2S alleviated gentamicin-induced nephrotoxicity by reducing reactive oxygen species (ROS)-mediated apoptosis in normal rat kidney-52E cells. We demonstrated that H 2S significantly improved the kidney structure and function of CRF rats. We found that H 2S decreased the protein levels of Bax, Caspase-3, and Cleaved-caspase-3, but increased the expression of Bcl-2. Treatment with H 2S reduced the levels of malondialdehyde and ROS and increased the activities of superoxide dismutase and glutathione peroxidase. H 2S significantly abolished the phosphorylation of extracellular signal-regulated protein kinase 1/2, c-Jun N-terminal kinase, and p38 in the kidney of CRF rats. Furthermore, H 2S decreased the expression levels of tumor necrosis factor-α, interleukin (IL)-6, IL-10, and monocyte chemoattractant protein-1, as well as the protein levels of p50, p65, and p-p65 in the kidney of CRF rats. In conclusion, H 2S could ameliorate adenine-induced CRF in rats by inhibiting apoptosis and inflammation through ROS/mitogen-activated protein kinase and nuclear factor-kappa B signaling pathways.
Related collections
Most cited references51
- Record: found
- Abstract: found
- Article: not found
Hydrogen sulfide-based therapeutics: exploiting a unique but ubiquitous gasotransmitter.
- Record: found
- Abstract: found
- Article: not found
Cystathionine γ-lyase deficiency mediates neurodegeneration in Huntington's disease.
- Record: found
- Abstract: found
- Article: not found