- Record: found
- Abstract: found
- Article: not found
Hsa-miR-24-3p Increases Nasopharyngeal Carcinoma Radiosensitivity by Targeting Both the 3’UTR and 5’UTR of Jab1/CSN5
Read this article at
Abstract
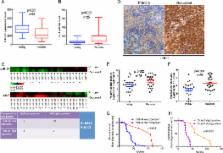
Radiotherapy is the standard therapy for nasopharyngeal carcinoma (NPC); however, radioresistance can hinder successful treatment. Here, we report that miR-24 acts as a tumor suppressor and radiosensitizer in NPC cells and xenografts by targeting Jab1/CSN5. Although accumulating evidence has shown that Jab1/CSN5 functions as an oncoprotein in human cancers, its regulation through miRs has not been described. In this study, we found that Jab1/CSN5 functioned in a manner opposite that of miR-24 in NPC tumorigenesis and radioresistance. We demonstrated that miR-24 inhibits Jab1/CSN5 translation via direct binding to its 3’UTR and 5’UTR, leading to tumor growth inhibition, and sensitizes NPC tumors to radiation in vivo. Furthermore, silencing Jab1/CSN5 phenocopied the function of miR-24 in NPC cells after ionizing radiation treatment, resulting in increased apoptosis. Finally, we analyzed 50 paired samples of primary and matched recurrent NPC tissues from 25 NPC patients and subjected them to high-throughput genomic quantitative nuclease protection assay for quantifying simultaneously miR and mRNA levels. Our results showed that miR-24 levels were significantly decreased in recurrent NPC and that levels of Jab1/CSN5, as its target, were higher than those in primary NPC. Together, our findings indicate that miR-24 inhibits NPC tumor growth and increases NPC radiosensitivity by directly regulating Jab1/CSN5 and that both miR-24 and Jab1/CSN5 can serve as prognostic markers for NPC recurrence; this, in turn, may provide a promising therapeutic strategy for reversing NPC radioresistance.
Related collections
Most cited references35
- Record: found
- Abstract: found
- Article: not found
Targeting microRNAs in cancer: rationale, strategies and challenges.
- Record: found
- Abstract: found
- Article: not found
MicroRNA-10a binds the 5'UTR of ribosomal protein mRNAs and enhances their translation.
- Record: found
- Abstract: found
- Article: not found
