- Record: found
- Abstract: found
- Article: found
The RELIEF study: Tolerability and efficacy of preservative-free latanoprost in the treatment of glaucoma or ocular hypertension
Read this article at
Abstract
Purpose:
To assess tolerability and efficacy following a switch from benzalkonium chloride–latanoprost to preservative-free latanoprost in patients with glaucoma or ocular hypertension.
Methods:
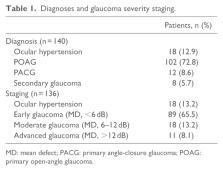
A total of 140 patients with glaucoma or ocular hypertension controlled with benzalkonium chloride-latanoprost for at least 3 months were switched to treatment with preservative-free latanoprost. Assessments were made on days 15, 45, and 90 (D15, D45, and D90) and included best-corrected visual acuity, intraocular pressure, slit lamp examination, fluorescein staining, tear film break-up time, patient symptom evaluation, and subjective estimation of tolerability.
Results:
Mean best-corrected visual acuity remained unchanged during the study. Mean intraocular pressure compared with baseline (D0) remained stable throughout the study (D0, 15.9 mmHg (standard deviation = 2.6); D90, 15.3 mmHg (standard deviation = 2.4); p < 0.006). Tear film break-up time improved or remained unchanged relative to baseline in 92% of patients at D45 and in 93% at D90. Moderate-to-severe conjunctival hyperemia was seen in 56.8% of patients at D0, but this figure decreased to 13.7%, 2.2%, and 1.6% at D15, D45, and D90, respectively. Subjective assessment of tolerability (0–10 scale) indicated improvement with change of therapy (mean score: 5.3 (standard deviation = 2.2) at D0 versus 1.9 (standard deviation = 1.7) at D90; p < 0.0001).
Related collections
Most cited references20
- Record: found
- Abstract: found
- Article: not found
Preservatives in eyedrops: the good, the bad and the ugly.
- Record: found
- Abstract: found
- Article: not found
Adverse effects of topical antiglaucoma medication. II. The outcome of filtration surgery.
- Record: found
- Abstract: found
- Article: not found
