- Record: found
- Abstract: found
- Article: found
Ebola Virus Binding to Tim-1 on T Lymphocytes Induces a Cytokine Storm
Read this article at
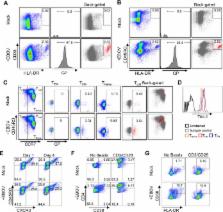
ABSTRACT
Ebola virus (EBOV) disease (EVD) results from an exacerbated immunological response that is highlighted by a burst in the production of inflammatory mediators known as a “cytokine storm.” Previous reports have suggested that nonspecific activation of T lymphocytes may play a central role in this phenomenon. T-cell immunoglobulin and mucin domain-containing protein 1 (Tim-1) has recently been shown to interact with virion-associated phosphatidylserine to promote infection. Here, we demonstrate the central role of Tim-1 in EBOV pathogenesis, as Tim-1 −/− mice exhibited increased survival rates and reduced disease severity; surprisingly, only a limited decrease in viremia was detected. Tim-1 −/− mice exhibited a modified inflammatory response as evidenced by changes in serum cytokines and activation of T helper subsets. A series of in vitro assays based on the Tim-1 expression profile on T cells demonstrated that despite the apparent absence of detectable viral replication in T lymphocytes, EBOV directly binds to isolated T lymphocytes in a phosphatidylserine–Tim-1-dependent manner. Exposure to EBOV resulted in the rapid development of a CD4 Hi CD3 Low population, non-antigen-specific activation, and cytokine production. Transcriptome and Western blot analysis of EBOV-stimulated CD4 + T cells confirmed the induction of the Tim-1 signaling pathway. Furthermore, comparative analysis of transcriptome data and cytokine/chemokine analysis of supernatants highlight the similarities associated with EBOV-stimulated T cells and the onset of a cytokine storm. Flow cytometry revealed virtually exclusive binding and activation of central memory CD4 + T cells. These findings provide evidence for the role of Tim-1 in the induction of a cytokine storm phenomenon and the pathogenesis of EVD.
IMPORTANCE
Ebola virus infection is characterized by a massive release of inflammatory mediators, which has come to be known as a cytokine storm. The severity of the cytokine storm is consistently linked with fatal disease outcome. Previous findings have demonstrated that specific T-cell subsets are key contributors to the onset of a cytokine storm. In this study, we investigated the role of Tim-1, a T-cell-receptor-independent trigger of T-cell activation. We first demonstrated that Tim-1-knockout (KO) mice survive lethal Ebola virus challenge. We then used a series of in vitro assays to demonstrate that Ebola virus directly binds primary T cells in a Tim-1–phosphatidylserine-dependent manner. We noted that binding induces a cytokine storm-like phenomenon and that blocking Tim-1–phosphatidylserine interactions reduces viral binding, T-cell activation, and cytokine production. These findings highlight a previously unknown role of Tim-1 in the development of a cytokine storm and “immune paralysis.”
Related collections
Most cited references63
- Record: found
- Abstract: found
- Article: not found
TIM genes: a family of cell surface phosphatidylserine receptors that regulate innate and adaptive immunity.
- Record: found
- Abstract: found
- Article: not found
Pathogenesis of Ebola hemorrhagic fever in cynomolgus macaques: evidence that dendritic cells are early and sustained targets of infection.
- Record: found
- Abstract: found
- Article: not found