- Record: found
- Abstract: found
- Article: found
Report From the International Society of Urological Pathology (ISUP) Consultation Conference on Molecular Pathology of Urogenital Cancers : IV: Current and Future Utilization of Molecular-Genetic Tests for Testicular Germ Cell Tumors
Read this article at
Abstract
Supplemental Digital Content is available in the text.
Abstract
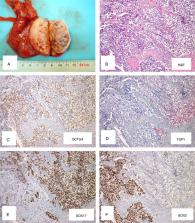
The International Society of Urological Pathology (ISUP) organized a Consultation Conference in March 2019 dealing with applications of molecular pathology in Urogenital Pathology, including testicular tumors (with a focus on germ cell tumors [GCTs]), preceded by a survey among its members to get insight into current practices in testicular germ cell tumor (TGCT) diagnostics and adoption of the ISUP immunohistochemical guidelines published in 2014. On the basis of the premeeting survey, the most commonly used immunomarker panel includes OCT3/4, placental alkaline phosphate, D2-40, SALL4, CD117, and CD30 for GCTs and the documentation of germ cell neoplasia in situ (GCNIS). Molecular testing, specifically 12p copy gain, is informative to distinguish non-GCNIS versus GCNIS related GCTs, and establishing germ cell origin of tumors both in the context of primary and metastatic lesions. Other molecular methodologies currently available but not widely utilized for TGCTs include genome-wide and targeted approaches for specific genetic anomalies, P53 mutations, genomic MDM2 amplification, and detection of the p53 inactivating miR-371a-3p. The latter also holds promise as a serum marker for malignant TGCTs. This manuscript provides an update on the classification of TGCTs, and describes the current and future role of molecular-genetic testing. The following recommendations are made: (1) Presence of GCNIS should be documented in all cases along with extent of spermatogenesis; (2) Immunohistochemical staining is optional in the following scenarios: identification of GCNIS, distinguishing embryonal carcinoma from seminoma, confirming presence of yolk sac tumor and/or choriocarcinoma, and differentiating spermatocytic tumor from potential mimics; (3) Detection of gain of the short arm of chromosome 12 is diagnostic to differentiate between non-GCNIS versus GCNIS related GCTs and supportive to the germ cell origin of both primary and metastatic tumors.
Related collections
Most cited references98
- Record: found
- Abstract: found
- Article: not found
Testicular germ-cell tumours in a broader perspective.
- Record: found
- Abstract: found
- Article: not found
POU5F1 (OCT3/4) identifies cells with pluripotent potential in human germ cell tumors.

- Record: found
- Abstract: found
- Article: found