- Record: found
- Abstract: found
- Article: found
Activity of Smurf2 Ubiquitin Ligase Is Regulated by the Wnt Pathway Protein Dishevelled
Read this article at
Abstract
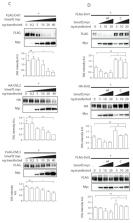
Wnt and BMP signaling pathways are two key molecular machineries regulating development and homeostasis. The efficient coordination of Wnt and BMP is essential in many developmental processes such as establishment of antero-posterior and dorso-ventral body axis, regulation of convergent extension, or development of various organ systems. SMAD ubiquitination regulatory factor (Smurf) family of E3 ubiquitin ligases are important and evolutionary conserved regulators of TGF-β/BMP signaling pathways. Smurf2 has been previously shown to regulate Wnt/planar cell polarity (PCP) signaling pathway by ubiquitinating Prickle1, one of the key components of PCP. We explored the role of Smurf2 in Wnt pathways in further detail and identified that Smurf2 is also a ubiquitin ligase of Dishevelled (DVL), the key cytoplasmic signal transducer in the Wnt pathway. Interestingly, the Smurf2 and DVL relationship expands beyond substrate-E3 ligase. We can show that DVL activates Smurf2, which allows Smurf2 to ubiquitinate its substrates from Wnt/PCP (Prickle1) as well as TGF-β/BMP (Smad2) pathways more efficiently. Using SMAD7 as an example of Smurf2 activator we show that DVL and SMAD7 both activates Smurf2 activity. In HEK293 cells the deficiency of DVL phenocopies absence of Smurf2 and leads to the increased phosphorylation of R-Smads. Smurf2-DVL connection provides a novel and intriguing point of crosstalk for Wnt and BMP pathways.
Related collections
Most cited references38
- Record: found
- Abstract: found
- Article: not found
Dishevelled: The hub of Wnt signaling.
- Record: found
- Abstract: found
- Article: not found
Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation.
- Record: found
- Abstract: found
- Article: not found