- Record: found
- Abstract: found
- Article: found
Peak Inspiratory Flow Rate in COPD: An Analysis of Clinical Trial and Real-World Data
Abstract
Background
The influence of peak inspiratory flow (PIF) on dose delivery from dry powder inhalers (DPIs) and association with treatment efficacy in patients with chronic obstructive pulmonary disease (COPD) has not been fully determined. In vitro studies have demonstrated adequate dose delivery through ELLIPTA DPI at PIF ≥30 L/min. This analysis of two clinical trials and a real-world population of COPD patients determined spirometric PIF distribution, and explored the relationship between PIF and outcomes in the trials.
Methods
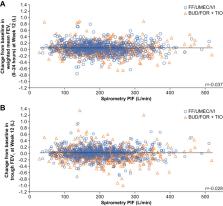
The replicate Phase IV, 12-week, randomized, double-blind 207608/207609 (NCT03478683/NCT03478696) trials evaluated fluticasone furoate/umeclidinium/vilanterol via ELLIPTA DPI versus budesonide/formoterol+tiotropium in COPD patients. This post hoc analysis assessed spirometric PIF distribution at screening and relationship between PIF and lung function outcomes in the pooled 207608/207609 population. Spirometric PIF distributions in a real-world population of COPD patients were evaluated by retrospective analysis of the Kaiser Permanente Northwest (KPNW) database to assess similarities between clinical trial and real-world populations.
Results
A total of 1460 (207608/207609) and 3282 (KPNW) patients were included. There was considerable overlap between spirometric PIF distributions for both populations. Overall, 99.7% and 99.8% of the 207608/207609 and KPNW populations, respectively, reported spirometric PIF ≥50 L/min, estimated as equivalent to ELLIPTA PIFR ≥30 L/min. In the 207608/207609 combined analysis, there was no significant interaction between spirometric PIF and treatment for lung function endpoints, indicating treatment effect is independent of PIF.
Conclusion
Nearly all COPD patients in the 207608/207609 and KPNW populations achieved spirometric PIF values estimated as equivalent to PIFR of ≥30 L/min through the ELLIPTA DPI. Lack of correlation between spirometric PIF at screening and treatment efficacy aligns with consistent dose performance from the ELLIPTA DPI across a wide range of PIFs, achieved by patients with COPD of all severities.
Most cited references35
- Record: found
- Abstract: found
- Article: not found
Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement
- Record: found
- Abstract: found
- Article: not found
Objective Assessment of Adherence to Inhalers by Patients with Chronic Obstructive Pulmonary Disease.

- Record: found
- Abstract: found
- Article: found