- Record: found
- Abstract: found
- Article: found
Development of an enhanced health-economic model and cost-effectiveness analysis of tiotropium + olodaterol Respimat ® fixed-dose combination for chronic obstructive pulmonary disease patients in Italy
Read this article at
Abstract
Background:
The objective of this study was to compare the cost-effectiveness of the fixed-dose combination (FDC) of tiotropium + olodaterol Respimat ® FDC with tiotropium alone for patients with chronic obstructive pulmonary disease (COPD) in the Italian health care setting using a newly developed patient-level Markov model that reflects the current understanding of the disease.
Methods:
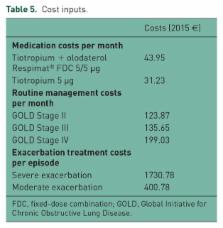
While previously published models have largely been based around a cohort approach using a Markov structure and GOLD stage stratification, an individual-level Markov approach was selected for the new model. Using patient-level data from the twin TOnado trials assessing Tiotropium + olodaterol Respimat ® FDC versus tiotropium, outcomes were modelled based on the trough forced expiratory volume (tFEV 1) of over 1000 patients in each treatment arm, tracked individually at trial visits through the 52-week trial period, and after the trial period it was assumed to decline at a constant rate based on disease stage. Exacerbation risk was estimated based on a random-effects logistic regression analysis of exacerbations in UPLIFT. Mortality by age and disease stage was estimated from an analysis of TIOSPIR trial data. Cost of bronchodilators and other medications, routine management, and costs of treatment for moderate and severe exacerbations for the Italian setting were included. A cost-effectiveness analysis was conducted over a 15-year time horizon from the perspective of the Italian National Health Service.
Results:
Aggregating total costs and quality-adjusted life years (QALYs) for each treatment cohort over 15 years and comparing tiotropium + olodaterol Respimat ® FDC with tiotropium alone, resulted in mean incremental costs per patient of €1167 and an incremental cost-effectiveness ratio (ICER) of €7518 per additional QALY with tiotropium + olodaterol Respimat ® FDC. The lung function outcomes observed for tiotropium + olodaterol Respimat ® FDC in TOnado drove the results in terms of slightly higher mean life-years (12.24 versus 12.07) exacerbation-free months (11.36 versus 11.32) per patient and slightly fewer moderate and severe exacerbations per patient-year (0.411 versus 0.415; 0.21 versus 0.24) versus tiotropium. Probabilistic sensitivity analyses showed tiotropium + olodaterol Respimat ® FDC to be the more cost-effective treatment in 95.2% and 98.4% of 500 simulations at thresholds of €20,000 and €30,000 per QALY respectively.
Related collections
Most cited references18
- Record: found
- Abstract: found
- Article: not found
Susceptibility to exacerbation in chronic obstructive pulmonary disease.

- Record: found
- Abstract: found
- Article: found
Lung function decline in COPD
- Record: found
- Abstract: found
- Article: not found