- Record: found
- Abstract: found
- Article: found
UV-Vis Spectrophotometry and Multivariate Calibration Method for Simultaneous Determination of Theophylline, Montelukast and Loratadine in Tablet Preparations and Spiked Human Plasma
Read this article at
Abstract
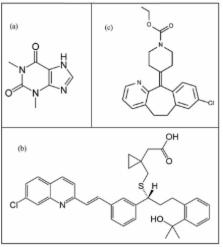
Resolution of binary mixtures of theophylline (THEO), montelukast (MKST) and loratadine (LORA) with minimum sample pre-treatment and without analyte separation has been successfully achieved by multivariate spectrophotometric calibration, together with partial least-squares (PLS-1), principal component regression (PCR) and hybrid linear analysis (HLA). Data of analysis were obtained from UV–Vis spectra of three compounds. The method of central composite design was used in the ranges of 2–14 and 3–11 mg L –1 for calibration and validation sets, respectively. The models refinement procedure and their validation were performed by cross-validation. The minimum root mean square error of prediction (RMSEP) was 0.173 mg L −1 for THEO with PCR, 0.187 mg L –1 for MKST with PLS1 and 0.251 mg L –1 for LORA with HLA techniques. The limit of detection was obtained 0.03, 0.05 and 0.05 mg L −1 by PCR model for THEO, MKST and LORA, respectively. The procedure was successfully applied for simultaneous determination of the above compounds in pharmaceutical tablets and human plasma. Notwithstanding the spectral overlapping among three drugs, as well as the intrinsic variability of the latter in unknown samples, the recoveries are excellent.
Related collections
Most cited references61
- Record: found
- Abstract: not found
- Article: not found
Comparison of multivariate calibration methods for quantitative spectral analysis
- Record: found
- Abstract: found
- Article: not found
Derivative spectrophotometry-recent applications and directions of developments.

- Record: found
- Abstract: found
- Article: found