- Record: found
- Abstract: found
- Article: found
Overlooked Mechanisms in Type 1 Diabetes Etiology: How Unique Costimulatory Molecules Contribute to Diabetogenesis
Read this article at
Abstract
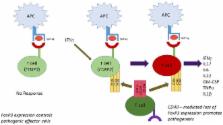
Type 1 Diabetes (T1D) develops when immune cells invade the pancreatic islets resulting in loss of insulin production in beta cells. T cells have been proven to be central players in that process. What is surprising, however, is that classic mechanisms of tolerance cannot explain diabetogenesis; alternate mechanisms must now be considered. T cell receptor (TCR) revision is the process whereby T cells in the periphery alter TCR expression, outside the safety-net of thymic selection pressures. This process results in an expanded T cell repertoire, capable of responding to a universe of pathogens, but limitations are that increased risk for autoimmune disease development occurs. Classic T cell costimulators including the CD28 family have long been thought to be the major drivers for full T cell activation. In actuality, CD28 and its family member counterparts, ICOS and CTLA-4, all drive regulatory responses. Inflammation is driven by CD40, not CD28. CD40 as a costimulus has been largely overlooked. When naïve T cells interact with antigen presenting cell CD154, the major ligand for CD40, is induced. This creates a milieu for T cell (CD40)–T cell (CD154) interaction, leading to inflammation. Finally, defined pathogenic effector cells including TH40 (CD4 +CD40 +) cells can express FOXP3 but are not Tregs. The cells loose FOXP3 to become pathogenic effector cells. Each of these mechanisms creates novel options to better understand diabetogenesis and create new therapeutic targets for T1D.
Related collections
Most cited references114
- Record: found
- Abstract: found
- Article: not found
TIM genes: a family of cell surface phosphatidylserine receptors that regulate innate and adaptive immunity.
- Record: found
- Abstract: found
- Article: not found
