- Record: found
- Abstract: found
- Article: found
The enzyme mechanism of patchoulol synthase
Read this article at
Abstract
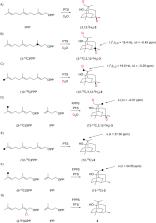
Different mechanisms for the cyclisation of farnesyl pyrophosphate to patchoulol by the patchoulol synthase are discussed in the literature. They are based on isotopic labelling experiments, but the results from these experiments are contradictory. The present work reports on a reinvestigation of patchoulol biosynthesis by isotopic labelling experiments and computational chemistry. The results are in favour of a pathway through the neutral intermediates germacrene A and α-bulnesene that are both reactivated by protonation for further cyclisation steps, while previously discussed intra- and intermolecular hydrogen transfers are not supported. Furthermore, the isolation of the new natural product (2 S,3 S,7 S,10 R)-guaia-1,11-dien-10-ol from patchouli oil is reported.