- Record: found
- Abstract: found
- Article: found
Different Chondrogenic Potential among Human Induced Pluripotent Stem Cells from Diverse Origin Primary Cells
Read this article at
Abstract
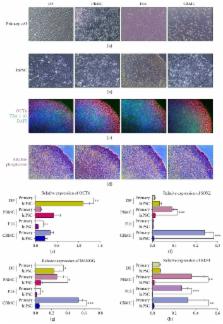
Scientists have tried to reprogram various origins of primary cells into human induced pluripotent stem cells (hiPSCs). Every somatic cell can theoretically become a hiPSC and give rise to targeted cells of the human body. However, there have been debates on the controversy about the differentiation propensity according to the origin of primary cells. We reprogrammed hiPSCs from four different types of primary cells such as dermal fibroblasts (DF, n = 3), peripheral blood mononuclear cells (PBMC, n = 3), cord blood mononuclear cells (CBMC, n = 3), and osteoarthritis fibroblast-like synoviocytes (OAFLS, n = 3). Established hiPSCs were differentiated into chondrogenic pellets. All told, cartilage-specific markers tended to express more by the order of CBMC > DF > PBMC > FLS. Origin of primary cells may influence the reprogramming and differentiation thereafter. In the context of chondrogenic propensity, CBMC-derived hiPSCs can be a fairly good candidate cell source for cartilage regeneration. The differentiation of hiPSCs into chondrocytes may help develop “cartilage in a dish” in the future. Also, the ideal cell source of hiPSC for chondrogenesis may contribute to future application as well.
Related collections
Most cited references42
- Record: found
- Abstract: found
- Article: not found
Epigenetic memory and preferential lineage-specific differentiation in induced pluripotent stem cells derived from human pancreatic islet beta cells.
- Record: found
- Abstract: found
- Article: not found
Incomplete DNA methylation underlies a transcriptional memory of somatic cells in human iPS cells.
- Record: found
- Abstract: found
- Article: not found