- Record: found
- Abstract: found
- Article: found
TET3 Mediates 5hmC Level and Promotes Tumorigenesis by Activating AMPK Pathway in Papillary Thyroid Cancer
Read this article at
Abstract
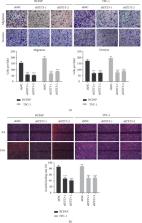
Thyroid cancer is the most common endocrine malignant tumor. The accurate risk stratification and prognosis assessment is particularly important for patients with thyroid cancer, which can reduce the tumor recurrence rate, morbidity, and mortality effectively. DNA methylation is one of the most widely studied epigenetic modifications. Many studies have shown that 5hmC-mediated demethylation played an important role in tumors. The hydroxylation of 5mC is catalyzed by ten-eleven translocation dioxygenase (TET). In this study, we first found that the abnormal expression of 5hmC was closely related to microcarcinoma, multifocal, extraglandular invasion and lymph node metastasis of thyroid carcinoma. Then, we identified TET3 was differentially expressed in thyroid cancers and normal tissues from the TET family. TET3 can promote the proliferation, migration, and invasion of thyroid cancer. TET3-mediated 5hmC can regulate the transcription of AMPK pathway-related genes to activate the AMPK pathway and autophagy and therefore promote PTC proliferation. These findings provide a preclinical rationale for the design of novel therapeutic strategies for this target to improve the clinical outcome of patients with PTC.
Related collections
Most cited references44
- Record: found
- Abstract: found
- Article: not found
Cancer statistics, 2020
- Record: found
- Abstract: found
- Article: not found
AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1.
- Record: found
- Abstract: found
- Article: not found