- Record: found
- Abstract: found
- Article: found
Alpha1a-Adrenoceptor Genetic Variant Triggers Vascular Smooth Muscle Cell Hyperproliferation and Agonist Induced Hypertrophy via EGFR Transactivation Pathway
Read this article at
Abstract
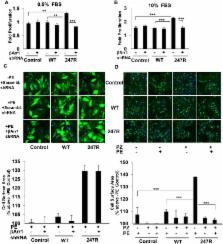
α 1a Adrenergic receptors (α 1aARs) are the predominant AR subtype in human vascular smooth muscle cells (SMCs). α 1aARs in resistance vessels are crucial in the control of blood pressure, yet the impact of naturally occurring human α 1aAR genetic variants in cardiovascular disorders remains poorly understood. To this end, we present novel findings demonstrating that 3D cultures of vascular SMCs expressing human α 1aAR-247R (247R) genetic variant demonstrate significantly increased SMC contractility compared with cells expressing the α 1aAR-WT (WT) receptor. Stable expression of 247R genetic variant also triggers MMP/EGFR-transactivation dependent serum- and agonist-independent (constitutive) hyperproliferation and agonist-dependent hypertrophy of SMCs. Agonist stimulation reduces contractility Using pathway-specific inhibitors we determined that the observed hyperproliferation of 247R-expressing cells is triggered via β-arrestin1/Src/MMP-2/EGFR/ERK-dependent mechanism. MMP-2-specific siRNA inhibited 247R-triggered hyperproliferation indicating MMP-2 involvement in 247R-triggered hyperproliferation in SMCs. β-arrestin1-specific shRNA also inhibited 247R-triggered hyperproliferation but did not affect hypertrophy in 247R-expressing SMCs, indicating that agonist-dependent hypertrophy is independent of β-arrestin1. Our data reveal that in different cardiovascular cells the same human receptor genetic variant can activate alternative modulators of the same signaling pathway. Thus, our findings in SMCs demonstrate that depending on the type of cells expressing the same receptor (or receptor variant), different target-specific inhibitors could be used to modulate aberrant hyperproliferative or hypertrophic pathways in order to restore normal phenotype.
Related collections
Most cited references79
- Record: found
- Abstract: found
- Article: not found
Matrix Metalloproteinases in Vascular Remodeling and Atherogenesis: The Good, the Bad, and the Ugly
- Record: found
- Abstract: found
- Article: not found
Teaching old receptors new tricks: biasing seven-transmembrane receptors.
- Record: found
- Abstract: found
- Article: not found