- Record: found
- Abstract: found
- Article: not found
NECROTIZING ENTEROCOLITIS : The Search for a Unifying Pathogenic Theory Leading to Prevention
research-article
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
HISTORICAL PERSPECTIVE AND SCOPE
In Schaffer's 1965 issue, Diseases of the Newborn,
103
no mention is made of necrotizing enterocolitis (NEC). In the 1971 issue,
104
it is discussed only briefly. Although references are made to an entity resembling
NEC in the 19th and early 20th centuries,43, 89, 95 it generally was not recognized
as a disease that affects primarily premature infants until the 1950s and 1960s,12,
80, 99, 107 which attests to its relatively infrequent incidence or recognition prior
to modern neonatal intensive care.
During the past two decades, improvements in mechanical ventilatory support coupled
with prevention and treatment for pulmonary immaturity have significantly improved
the survival rate of low birth weight neonates. Concurrently, the incidence of NEC
has increased in most centers and has emerged as the most common gastrointestinal
emergency in neonates, affecting 2000 to 4000 newborns in the United States each year.21,
22, 65, 102, 116, 125 Of these, 10% to 50% die, resulting in approximately 1000 infant
deaths per year.
36
This number is close to the number of all US children fewer than 15 years of age who
die of leukemia, or all children and adolescents fewer than 20 years who die of meningitis.
The survivors of the acute episode of NEC frequently suffer with the effects of short
bowel syndrome,63, 64 which is a major cause of prolonged hospitalization and high
medical expenses.
Comprehensive information about the clinical aspects of NEC can be found in numerous
textbooks and previously written reviews. *
In this review, the clinical presentation and treatment of NEC is given only brief
attention. The major focus is to provide an in-depth discussion of putative pathophysiologic
events leading to NEC. An understanding of these events may hold the key for future
prevention of this devastating disease.
CLINICAL PRESENTATION
Necrotizing enterocolitis is characterized by the following symptomatology and pathology:
abdominal distention and tenderness, pneumatosis intestinalis, occult or frank blood
in stools, intestinal gangrene, bowel perforation, sepsis, and shock. Based on clinical
presentation, Bell and colleagues
11
originally described three levels of NEC, with stage 1 being suggestive, stage 2 being
definitive, and stage 3 being severe (Table 1)
. Although stage 1 is nonspecific and may, in many instances, reflect feeding intolerance,
sepsis, or gastrointestinal hemorrhage due to stress or other factors, stages 2 and
3 frequently are associated with considerable morbidity and mortality.
Table 1
MODIFIED BELL STAGING CRITERIA FOR NECROTIZING ENTEROCOLITIS
Stage
Classification<
Systemic Signs
Intestinal Signs
Radiologic Signs
IA
Suspected NEC
Temperature instability, apnea, bradycardia, lethargy
Increased pregavage residuals, midabdominal distention, emesis, guaiac-positive stool
Normal or intestinal dilation, mild ileus
IB
Suspected NEC
Same as above
Bright red blood from rectum
Same as above
IIA
Proven NEC—mildly ill
Same as above
Same as above, plus absent bowel sounds, with or without abdominal tenderness
Intestinal dilation, ileus, pneumatosis intestinalis
IIB
Proven NEC—moderately ill
Same as above, plus mild metabolic acidosis and mild thrombocytopenia
Same as above, plus absent bowel sounds, definite tenderness, with or without abdominal
cellulitis or right lower quadrant mass
Same as IIB, plus definite ascites
IIIA
Advanced NEC—severely ill, bowel intact
Same as IIB, plus hypotension bradycardia, severe apnea, combined respiratory and
metabolic acidosis, disseminated intravascular coagulation, and neutropenia
Same as above, plus signs of generalized peritonitis, marked tenderness, and distention
of abdomen
Same as IIB, plus definite ascites
IIIB
Advanced NEC—severely ill, bowel perforated
Same as IIIA
Same as IIIA
Same as IIB, plus pneumoperitoneum
NEC = necrotizing enterocolitis.
RADIOLOGIC FINDINGS
During the early stages of illness, the radiographic findings are nonspecific and
include dilated bowel loops, generalized bowel distention, and bowel-wall thickening.
A single persistent dilated loop should cause suspicion but is not a definitive sign
of NEC or perforation.
Pneumatosis intestinalis, or gas in the bowel wall, in the appropriate clinical setting
is usually diagnostic of NEC (Figs. 1
and 2)
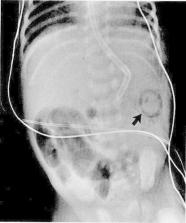
When pneumatosis intestinalis extends into the portal circulation (Fig. 3)
, it frequently is associated with severe disease. Pneumoperitoneum is frequently
diagnostic of intestinal perforation with NEC. The air within the peritoneal cavity
floats to the most nondependent area, which is just beneath the anterior upper abdominal
wall in the supine patient. This air frequently outlines the falciform ligament (Fig.
4).
Left lateral decubitus films can provide more information than supine films. Free
air floats to the right surface of the peritoneal cavity and appears as a lucent area
lateral to the liver edge. Careful serial reviews of left lateral decubitus films
commonly are used to follow the progression of NEC and to determine whether perforation
has occurred. Usually, pneumoperitoneum is considered an indication for surgery, whereas
pneumatosis alone, without clinical deterioration of hematologic signs or acid-base
status, usually is treated medically.
Figure 1
Several dilated air-filled loops of bowel in the right lower quadrant indicate a focal
ileus. The arrow points to submucosal air in the splenic flexure.
Figure 2
Film of the chest and abdomen shows massive air-distended bowel with diffuse intramural
air.
Figure 3
Air in the portal system (arrow).
Figure 4
Left lateral decubitus film of the abdomen shows free intraperitoneal air from a bowel
perforation. Free air is shown on both sides of the falciform ligament (arrow).
LABORATORY FEATURES
No individual laboratory features are diagnostic of NEC. Laboratory analysis primarily
is used to confirm clinical diagnostic impressions and to judge progression of severity
of disease.
108
Peripheral hematologic studies may reveal abnormally high or low white blood cell
counts with a shift toward immature precursors. Progressively decreasing absolute
granulocyte count and thrombocytopenia suggest increasing severity of disease. If
these are seen together with acidosis and severe electrolyte abnormalities, then the
severity of NEC likely is progressing toward the necessity for surgical intervention.
PATHOLOGIC FINDINGS
The pathologic findings of NEC have been described by examination of the most severely
affected patients who either died or who had intestinal perforation requiring resection
of gangrenous bowel.5, 65 Gross examination reveals involvement predominantly in the
terminal ileum and proximal colon. In severe cases, however, the bowel from the stomach
to the rectum may be involved. Histologic analysis reveals mucosal edema, hemorrhage,
coagulation necrosis, and mucosal ulceration. The pathologic changes of NEC suggest
a multifactorial cause, with bacterial overgrowth, inflammation, ischemia, and necrotic
tissue all having a role.
CURRENT THERAPIES
Therapy for NEC is highly dependent on its severity. Bell's staging criteria
11
can be highly useful as guidelines. In stage 1, there is only a strong suspicion of
the disease. Because of the potentially devastating progression of NEC, precautions
need to be taken. In this scenario, clinical judgment based on the patient's condition
should guide whether and how long the patient should be taken off enteral feedings,
whether and how long intravenous antibiotics should be used, and how aggressively
the patient is monitored with radiographs and laboratory tests. Once a definitive
diagnosis is made but the patient has not progressed to surgical NEC (usually stage
3), the bowel should be decompressed using a large-bore orogastric tube with low intermittent
suction. Careful attention needs to be placed on managing fluids and electrolytes
because considerable third spacing of fluids with losses of sodium and protein may
occur. Potassium fluxes (high or low serum potassium) with acid-base imbalances are
likely to occur. Renal failure is a frequent concomitant finding. Systemic antibiotic
therapy, usually with ampicillin and gentamicin, are started after obtaining blood
cultures. In cases in which resistant Staphylococcus epidermidis is suspected, vancomycin
is used instead of ampicillin. When perforation is suspected, or has occurred, clindamycin
or metronidazole is used to treat anaerobic infection. Intensive support of respiratory
and circulatory status is provided, as are frequent monitoring of the abdominal status
with left lateral decubitus radiographs and frequent monitoring of acid-base and hematologic
status. A persistent acidosis with continued deterioration of platelet and white blood
cell counts may be an indication for surgery even in the absence of overt perforation
on radiography.
A review of surgical therapy of NEC is beyond the scope of this review. It should
be remembered, however, that NEC is a disease that requires a carefully coordinated
approach among neonatologists and pediatric surgeons. Because NEC can be such a rapidly
progressive disease, the pediatric surgery section should be notified immediately
whenever a diagnosis of NEC is made, even when only medical intervention is necessary
at the time. This will allow for more rapid mobilization for surgery if and when it
progresses to a surgical emergency.
RISK FACTORS AND PUTATIVE PATHOPHYSIOLOGY
The pathophysiology of NEC is not clearly understood. Although classically described
as a disorder of sick premature infants in the neonatal intensive care unit (NICU),
clinical experience shows that this disease occurs in several different settings and
hosts. Although occasionally occurring in term infants and sick preterm infants who
are not on enteral feeding and on ventilatory support, many cases of NEC occur in
premature infants receiving intermediate or stepdown neonatal intensive care.29, 112
Extremely premature infants (<28 weeks' gestation) are at risk for the development
of NEC for a protracted period. The risk is high until the infant has achieved a postconceptual
age of 35 to 36 weeks and sometimes later depending on coincident gastrointestinal
problems.126, 127
The current thinking about the pathophysiology of NEC is based on epidemiologic studies
from which several important risk factors have been dissected. The putative risk factors
that predominate are prematurity, aggressive enteral feedings, infectious agents,
and hypoxicischemic insults.
PREMATURITY
The primary risk factor for NEC is prematurity because approximately 90% of cases
occur in premature infants.57, 65, 126 This disease rarely is seen in older children
and adults. The cause of this age restriction remains unclear, but an immature mucosal
barrier and immune response, in addition to impaired circulatory dynamics, are thought
to make premature neonates particularly susceptible.62, 83 Immaturities of the developing
gastrointestinal tract include immunologic factors, poor motility, reduced digestive
absorptive function, increased membrane fluidity, low levels of protective mucus,
and reduced regenerative capabilities, resulting in increased potential for tissue
damage. Differences in gastrointestinal maturity that predispose premature infants
to NEC include the following:
Immunologic factors
Decreased IgA secretory component
Decreased intestinal T lymphocytes
Poor antibody response
Lumenal factors
Lower H+ ion output in stomach
Low proteolytic enzyme activity
Immature intestinal barrier
Mucin blanket composition
Microvillus membrane biophysical properties and composition
Higher permeability
Lower and less organized motility
Immunologic Factors
Necrotizing enterocolitis occurs most frequently in the terminal ileum and colon.
66
Large numbers of lymph follicles (Peyer's patches) are present in these regions.
88
An observation in rabbits of decreased IgA secretory component overlying the Peyer's
patches of the ileum,
93
increased macromolecular uptake in this area60, 87, 129 and translocation of bacteria
in this region13, 14, 56 are interesting in view of the frequent localization of NEC
to this part of the intestine. Infant animals have also been shown to have decreased
numbers of intestinal T lymphocytes,
50
indicating a compromise in cellular immunity early in life. The underdeveloped T-lymphocyte
function may compromise immunologic surveillance and recognition of alterations in
the membrane of an epithelial cell infected by a bacterial or viral agent. This compromises
the ability to destroy the infected cell before the infectious agent can cross the
basement membrane underlying the epithelium.
59
Infants of less than 35 weeks' gestation have demonstrated a relatively poor response
in the production of antibodies when compared with those of more than 35 weeks' gestation.
97
As the microbial agents or their toxins are allowed to enter the intestine, the premature
newborn likely has a limited capability to respond immunologically.73, 97
Immature Lumenal Factors
Numerous immaturities in lumenal digestion exist in premature infants. One of the
first lines of defense against ingested pathogens and toxins is gastric acid.
119
Gastric-hydrogen ion output is low in the human neonate when compared with adults.48,
83 This places these infants at an increased risk for colonization of enteric pathogens.
Accordingly, acidification of feedings was demonstrated to decrease the incidence
of NEC in one study.
27
Proteolytic enzyme activity is also low. Enterokinase, the brushborder enzyme in the
duodenum, converts inactive trypsinogen to active trypsin. The relatively low enterokinase
activity4, 132 and subsequently low tryptic activity might suppress the hydrolysis
of various toxins that have the ability to damage the intestine. The occurrence of
pigbel, a disease similar to NEC, seen in New Guinea, supports the importance of proteases
as lumenal protective agents. Many highlanders of New Guinea subsist on a diet high
in antiproteases. During festivals, massive quantities of uncooked meat are consumed
after a prolonged fast. Enterocolitis develops, thought to be due to Clostridium beta
toxin,
82
which passes into the intestine without the benefit of hydrolysis by endogenous proteases.
The ensuing necrotic lesions in the gastrointestinal tract together with the propensity
to perforate are similar to the intestinal pathology of NEC.
Immature Intestinal Epithelial Barrier
The intestinal mucin blanket also seems to be scant and have a different composition
in newborn infant2, 57, 102 when compared with adults, which makes the immature intestine
more permeable to high molecular weight molecules.
39
This is also likely to facilitate bacterial adherence to the epithelium.2, 57, 101
The microvillus membrane composition and biophysical properties have been found to
change with increasing maturation. Neonatal rabbits'
90
and rats'
51
microvillus membranes have higher lipid-protein ratios than those of adults. The fluidity
(organization) of the microvillus membrane also changes as the animal matures and
is shifted toward a more mature pattern by glucocorticoids administered to the mother
antenatally or the infant postnatally.85, 91
Immature neonates have higher intestinal permeability than older children and adults.
Preterm infants, especially those born at less than 33 weeks of gestation have higher
serum concentrations of β-lactoglobulin than term neonates given equivalent milk feedings.
98
The permeability of the preterm human intestine to intact carbohydrate is greater
in infants than in children or adults.
7
Using measurements of lactulose and rhamnose in the urine after ingesting milk containing
these carbohydrates, some investigators have found that preterm neonates of 31 to
36 weeks' gestational age had an enhanced permeability of lactulose in the first week
of life that changed to a more mature pattern in the second week. Infants born at
a gestational age of 26 to 29 weeks had a more mature period of permeability at birth,
followed by a temporary period of enhanced permeability at 3 to 4 weeks of age. Overall,
there seems to be a developmental pattern of decreased permeability with maturation.
Breast-feeding is associated with a lower permeability to lactulose than formula-feeding,
120
which suggests that human milk contains factors that stimulate maturation of the small
intestine. The specific nature of these factors remains speculative.
In addition to being relatively permeable to the uptake of macromolecules, the intestine
of the newborn is also more permeable to the uptake of intact bacteria. When a breakdown
in the mucosal barrier occurs, lumenal bacteria may translocate across the bowel wall
into the blood or mesenteric lymph nodes.47, 121 Translocation is likely to be responsible
for many of the positive bacterial cultures obtained from the blood of neonates with
NEC.
34
Motility
The motility of the small intestine in premature infants is considerably lower and
less organized than that in term infants.
121
This is caused by an intrinsic immaturity of the enteric nervous system that may cause
delayed transit and subsequent bacterial overgrowth and distention. Although it is
not clear specifically what role immature motility has in the pathogenesis of NEC,
it likely contributes to the milieu in which the interaction of nutrients, immature
host defenses, and other factors initiate the cascade of events culminating in NEC.
15
AGGRESSIVE ENTERAL FEEDINGS
Necrotizing enterocolitis rarely, if ever, occurs in utero.21, 65, 72 The fetus swallows
up to 150 mL/kg/d of sterile amniotic fluid, which contains various nutrients, growth
factors, and immunoglobulins in concentrations very different than those of formula
or human milk.94, 106 Although amniotic fluid provides a small amount of nutrients
that can be utilized by the fetus, the majority of nutrition for the fetus comes from
the placental circulation. In postnatal life, the previously sterile intestine becomes
colonized with bacteria and is frequently stressed with relatively concentrated feedings.
What accounts for the exclusive onset of NEC in the postnatal state? Perhaps, some
of the components of amniotic fluid are highly protective to the fetal gastrointestinal
(GI) tract. The fact that the GI tract of the fetus is not colonized with bacteria
or exposed to a high volume of lumenal nutrients also likely has a role.
Although NEC occasionally occurs in infants who have never been fed,
3
it most frequently occurs in premature infants on enteral feedings and especially
those whose enteral intakes are being aggressively increased. The advancement of formula
feedings at rates greater than 20 kcal/kg/d has been found to be associated with an
increase in the incidence of NEC.3, 18, 20, 29, 118, 130 Despite the importance of
aggressive enteral feedings in the pathogenesis of NEC, the finding in several studies
that "minimal enteral feeding" or priming the GI tract by a very slow intake using
food as a trophic agent to stimulate GI mucosal development has not been associated
with an increased incidence of NEC.71, 76, 79, 111 Rather, there has been an improved
tolerance to subsequent enteral feedings, lower incidence of cholestasis, and higher
levels of potentially trophic gut hormones.
The possibility that human milk provides protection against NEC is supported by a
multicenter trial designed to evaluate the effect of early diet on the incidence of
NEC.
77
The study population, which was divided into infants fed only formula, those fed with
formula plus expressed human milk, and those fed with expressed human milk alone,
showed an incidence of 7.2%, 2.5%, and 1.2%, respectively. The theoretic basis of
this is that human milk contains several known growth factors,
96
hormones,
67
macrophages, leukocytes, lymphocytes, immunoglobulins,
75
and enzymes.
110
Whether a similar protective effect would be incurred by banked donor human milk,
which loses many potentially protective factors, remains speculative.
INFECTIOUS AGENTS
Prematurity and the presence of bacteria in the GI tract seem to be the most consistent
risk factors associated with the development of NEC. Whether bacteria are primary
inciting factors, merely permissive agents, or both in the pathogenic cascade leading
to NEC remains unanswered.
Several lines of evidence support th0e thesis that infection is necessary for the
development of NEC.10, 19, 72, 100 This includes epidemiologic evidence for outbreaks
suggestive of an infective process, the frequent isolation of infectious agents with
NEC, and the decreased incidence of NEC resulting from preventive measures. Because
many of the bacteria isolated from infants with NEC (from stool, blood, and peritoneal
fluid) are organisms commonly found in the intestine, it is impossible to tell whether
the presence of these bacteria is a causative factor or whether they are merely bystanders
to a separate pathologic process. The latter is unlikely because of the large number
of infants with NEC who exhibit overt pneumatosis intestinalis. If one uses Bell's
criteria for the diagnosis of "proven" NEC (stage 2), the radiologic finding of pneumatosis
is a necessary component. The gas in pneumatosis is thought to be derived primarily
from bacterial fermentation.43, 44, 61
Kosloske68, 69, presents bacteria as central factors in the "unifying hypothesis for
pathogenesis and prevention of necrotizing enterocolitis." Bacteria commonly isolated
from infants with NEC are gram-negative rods, including Klebsiella spp., Escherichia
coli Enterobacter spp., and Pseudomonas spp.
28
These bacteria can be isolated from blood, peritoneal fluid, intestinal tissues, and
feces.28, 81, 100, 122, 131 Other microorganisms associated with NEC are the bacteria
Clostridium difficile and Staphylococcus epidermidis and the viruses coronavirus and
rotavirus. The major arguments against etiologic roles for C difficile and S epidermis
in NEC are the high rate of isolation of these bacteria or identification of their
toxins in healthy, matched neonates and the lack of consistent recovery or detection
of the bacteria or toxins in patients with NEC.
69
Bacterial toxins also have been proposed as causes of NEC
105
however, some potent toxins, such as Clostridium toxin, are isolated commonly from
asymptomatic infants.
37
Because many different infectious agents have been associated with NEC, these agents
may possess common virulence factors that may predispose susceptible hosts to the
cascade of events involved in the pathophysiologic cascade of NEC. For example, many
members of the family Enterobacteriaceae possess genes encoding Shiga-like toxin and
cholera-like toxin.1, 53, 115 Whether these genes are expressed by NEC pathogens and
whether they have any role in disease are still unknown. Other possible common virulence
factors may involve metabolic traits that predispose the host to other factors in
the pathogenic cascade, such as a high capability to ferment lactose.
26
A majority of very premature babies in the NICU are started on broad-spectrum IV antibiotic
therapy shortly after birth during a "rule out sepsis" work-up. This can markedly
alter the normal flora with which the neonate would become colonized.9, 55 Rather
than becoming colonized with Lactobacillus and other "normal" gut flora, resistant
species indigenous to the NICU may colonize in the baby's intestine. Whether or how
much of a role this has in the pathogenesis of NEC is not known. Recent studies also
have shown that NEC-associated bacteria have a greater propensity than non-NEC-associated
bacteria of the same species to prevent adherence of gram-positive bacteria to the
GI tract and to cause disease in an animal model during coinfection with grampositive
isolates from the homologous child.
92
This could lead to an intestinal milieu consisting of a very large number of organisms
more conducive to the development of NEC than would otherwise reside there. Other
studies have shown that certain Klebsiella species that have a genetic predisposition
(plasmid) for active fermentation of lactose also have the ability to cause NEC in
isolated loops of rabbit small intestine.
26
Infection-Associated Inflammatory Mediators
Similar to sepsis and adult respiratory distress syndrome, NEC seems to involve a
final common pathway that includes the endogenous production of inflammatory mediators
involved in the development of intestinal injury. Endotoxin lipopolysaccharide, platelet-activating
factor (PAF), tumor necrosis factor (TNF), and other cytokines together with prostaglandins
and leukotrienes are thought to be involved in the final common pathway of NEC pathogenesis.25,
52
Bacteria possess endotoxins that instigate the inflammatory cascade by activating
PAF, TNF, and interleukin 1. PAF injected into the aorta of adult rats has been found
to cause necrosis of the bowel
24
that can be prevented by pretreatment with PAF-acetylhydrolase
70
and can be exacerbated by a nitric oxide synthase inhibitor.
78
The generation of these inflammatory mediators also has been shown to be decreased
by the pretreatment with glucocorticoids,
24
which also have been shown to decrease the incidence of NEC if administered to the
mother prior to the birth of her infant and in infants who are at high risk for the
development of NEC.
6
HYPOXIA-ISCHEMIA
Early theories of the pathogenesis of NEC suggested circulatory perturbations to be
the most important determinants of pathogenesis.74, 113 The physiologic support for
linking ischemia to NEC relates a shunting of blood from the intestine during times
of perinatal asphyxia. This was generated by the fact that many of the babies at that
time who developed NEC also had antecedent episodes of perinatal distress.54, 74,
113 Laboratory investigations in several animal models also demonstrated that a lack
of perfusion to the bowel was an important antecedent to bowel necrosis.30, 31, 117
This, coupled with the knowledge of a known redistribution of blood from the intestine
to preserve blood flow to the brain, heart, and kidneys (in diving mammals, the "diving
reflex"), led to the logical conclusion that hypoxia-ischemia is a major predisposing
factor in the pathogenesis of NEC.
109
Other compelling evidence supporting ischemia as a major causative factor for NEC
included histopathologic data that clearly indicated that ischemia occurs in the disease
process.
5
The clinical data that supported a hypoxic-ischemic role in the pathogenesis of NEC
were largely anecdotal. The physiologic studies and clinical reports linking circulatory
disturbances to the etiology of NEC recently have been disputed by both physiologic
and clinical data.
86
Subsequent epidemiologic case-control studies showed that hypoxicischemic insults
were not relevant risk factors in the development of NEC.29, 33, 65, 114 The fact
that so many cases of NEC occur in the intermediate or stepdown intensive care unit
in babies who have never had known antecedent stresses, such as low Apgar scores,
need for ventilatory support, umbilical catheters, polycythemia, or significant apneic
episodes mitigates against hypoxia-ischemia as a major causative factor.
Even though both physiologic and clinical observations argue against a primary circulatory
aberration as the cause of NEC, the histologic appearance of the disease, which includes
coagulation necrosis suggesting ischemia as a component of the pathway, is compelling.
5
Hypoxia/ischemia to the bowel actually may be a secondary event that is mediated by
other factors. It is tenable that inflammatory mediators released after endotoxin
or bacterial invasion across the mucosa may lead to vasoconstriction and hypoxic-ischemic
insults. The vascular control systems are known to be quite immature in these infants
and could make the premature intestine more susceptible to the development of local
tissue hypoxia.
86
Resting vascular resistance is known to be low in premature infants. The endothelium
of the small intestine is responsible for the production of nitric oxide, a potent
mediator known to maintain low resting vascular resistance. If endothelial injury
occurs, subsequent damage to the nitric oxide system likely could have a detrimental
effect on local vascular tone, which could, at least theoretically, result in some
of the tissue injury seen in NEC that is compatible with an ischemic insult.
78
Thus, other triggers, such as bacterial toxins or chemical irritation, could set off
a cascade of events that could lead to endothelial disruption, altered nitric oxide
production, and subsequently vascular compromise.
86
The specific relationship of endotoxin, PAF, and nitric oxide together with other
mediators remains to be elucidated. Whether elucidation of this pathway might result
in a potential treatment or prevention for NEC is discussed later.
PREVENTION
Several measures are commonly used to prevent NEC outbreaks. The first includes careful
epidemic precautions when an outbreak of NEC is suspected. These precautions are predicated
on an increased awareness of NEC being present in the NICU in more than one infant
and a suspicion that microbial agents are involved in the pathogenesis of the outbreak.
Preventive measures include strict infection-control measures to prevent fecal and
oral spread; cohorting of patients, contacts, and personnel; and using a decreased
threshold for early intervention, such as placing babies nulla per os (NPO), providing
antibiotics while cultures are pending, and delaying or slowing enteral feedings while
an outbreak is suspected. Although oral antimicrobial agents have been used for prophylaxis
in contacts to interrupt the outbreak,8, 40, 49 the possibility of emergence of resistant
organisms limits their routine long-term use.
41
Although glucocorticoids are not routinely used for the prophylaxis of NEC as they
are being used for the prevention of respiratory distress syndrome (RDS), the data
are highly suggestive of their potential benefit.6, 32, 51, 58 The mechanism for this
is unclear but may involve a maturational effect on the microvillus membrane,85, 91
an increase in the activities of several enzymes involved in digestion and absorption
of nutrients,
83
or a decrease in mucosal inflammation. In reality, many of the babies at highest risk
for NEC are also at highest risk for RDS and thus may actually be receiving prophylaxis
antenatally with glucocorticoids via the mother. The decreased incidence of NEC in
babies of mothers who are treated with antenatal steroids6, 32 should provide an additional
indication for the administration of corticosteroids to mothers in preterm labor.
Whether the routine use of postnatal glucocorticoid prophylaxis for NEC would be effective
without causing other risks to the infant, such as increasing the incidence of sepsis
or catabolism, is not known, and further studies are needed to determine the risks
versus the benefits of such prophylaxis.
Providing human milk to premature infants has been shown to decrease the incidence
of NEC in one large multicenter study.
77
Fresh human milk is composed of numerous immunoprotective factors, such as immunoglobulins,
lysozyme, lactoferrin, macrophages, lymphocytes, and neutrophils.
23
The possibility of banked or refrigerated donor milk providing benefit is less clear
because the cellular components and immunoglobulins are compromised by the pasteurization
or freezing process. Another potentially beneficial component of human milk recently
discovered is PAF acetyl hydrolase (PAF-AH-102). This enzyme inhibits the activity
of PAF, which is likely to be a significant mediator in the pathophysiologic cascade
of NEC. Because of the likely protective effect against NEC and other infections in
addition to the nutritional and psychosocial benefits afforded by human milk, it is
the author's opinion that premature infants should be provided with his or her own
mother's fresh milk whenever feasible.
The finding that an IgG-IgA preparation obtained from human serum provided protection
against NEC in premature infants in a randomized trial is compelling.
42
Whether this preparation acts to directly prevent bacterial invasion into the gut
or to counteract the release of cytokines from monocytes
128
is not known. This study should be repeated and confirmed at a larger scale, preferably
in a multicenter format prior to recommending routine use of this modality for the
prevention of NEC.
As previously discussed, the aggressive institution of enteral feedings in preterm
infants is a frequent risk factor associated with NEC. This has, in many cases, caused
neonatologists to institute an overly cautious approach to enteral feedings whereby
the baby is placed NPO for several weeks after birth and nourished only by the parenteral
route. This approach is known to cause atrophy of the intestinal mucosa and may be
highly detrimental in that the onset of NEC may be delayed only after feedings are
finally instituted. An alternative approach with several different names, including
"minimal enteral nutrition," "intestinal priming," and "hypocaloric feedings," has
been studied in a controlled randomized manner38, 79 involving use of enteral nutrition
to provide topical nutrition to the small intestine during the first week or two of
life while providing most of the nutritional needs for the infant by the parenteral
route. It is usually reserved for infants weighing less than 1500 grams. "Priming"
is not contraindicated while the infant is on ventilatory support or using umbilical
catheters. The enteral feedings are increased so that the infant is on full enteral
intake in the third to fourth week of life. This approach has been found to improve
tolerance to subsequent enteral intake, stimulate early secretion of several intestinal
hormones that may be trophic in the intestinal mucosa, and improve motility.
16
Despite demonstrating these advantages, the numbers from the individual studies were
too small to determine whether this approach caused a significant decrease in the
incidence of NEC.
The osmolarity, carbohydrate composition, and pH of the enteral intake also have been
implicated in the pathogenesis of NEC. Formulas with high osmolarity have been shown
to increase the incidence of NEC in human infants
17
and cause mucosal injury in animal models.
35
Many oral medications, such as vitamin preparations, use hyperosmolar vehicles123,
124 that potentially could cause osmotic injury to the bowel. The carbohydrate found
in human milk and most commercial infant formulas is lactose. There is some controversy
as to whether the premature small intestine has the capability to hydrolyze lactose
to the same degree as that of the term infant.
83
Also, the lactose not hydrolyzed by the intestine may be hydrolyzed by bacteria, with
the subsequent fermentative generation of hydrogen gas and short-chain fatty acids.
61
The presence of certain bacteria that have an especially efficient capability to ferment
lactose using beta-galactosidase could lead to a high concentration of lumenal short-chain
fatty acids, which could alter the pH of the small bowel and cause mucosal breakdown,
with the invasion of bacteria into the mucosa and production of pneumatosis intestinalis.
26
In the upper GI tract, the situation is different than in the distal. Many premature
infants are hypochlorhydric and do not respond as quickly as older children or adults
to a meal with brisk production of gastric acid and peptic proteases.48, 83 This acid
environment is an effective barrier to many potentially pathogenic microorganisms.
A prospective doubleblind study that compared the incidence of NEC in a group of infants
fed a regular formula to infants fed a formula supplemented with acid to achieve a
pH between 3 and 4 showed a lower incidence of NEC in the acid-supplemented group.
27
These studies should be confirmed before considering routine use of this method.
Another method that has been attempted as prophylaxis against NEC included the provision
of oral aminoglycosides (kanamycin or gentamicin). Several of the studies suggested
a significant decrease in the incidence of NEC in the groups given oral antibiotics,8,
40, 49 compared with controls. The long-term use of these antibiotics unfortunately
was associated with the emergence of resistant strains of Staphylococcus, Klebsiella,
and E coli.
41
These findings have discouraged the routine use of long-term oral antibiotic therapy
for the prophylaxis of NEC.
(Table 2)
shows the pathophysiologic features together with the corresponding preventative measures
that have been attempted or are being used in the prevention of NEC.
Table 2
PREVENTION BASED ON PATHOPHYSIOLOGY
Pathophysiologic Feature
Preventive Measures
Microbial infection
Oral antibiotics Formula acidification Epidemiologic control Human milk IgG-IgA
Immature intestine
Glucocorticoids Human milk Intestinal priming
FUTURE APPROACHES FOR PREVENTION
As the pathophysiologic cascade for NEC becomes more defined and the tools for investigative
science improve, the likelihood of finding effective prophylaxis for NEC increases.
The potential for prophylaxis of NEC can be found at several levels. It is reasonable
to assume that interventions aimed at the more proximal events of the cascade offer
a greater likelihood for effective prophylaxis. Areas for possible intervention include
the following:
Identification of a common microbial agent or common bacterial virulence factors
Maturation of the intestine using trophic agents
Interference in the host-response to the inciting factors
At this time, a single microbial agent known to cause NEC has not been identified.
Some as yet unidentified microbes may be involved. Viruses such as coronavirus and
rotavirus have been implicated in the pathogenesis of NEC but have not been consistently
found. As previously discussed, it is highly likely that bacteria are involved, not
necessarily as triggering agents, but as agents that enable the pathophysiologic cascade
of NEC to proceed. The fact that so many premature neonates are placed on broad-spectrum
antibiotics shortly after birth to rule out sepsis changes the gut flora toward resistant
organisms that are likely to possess virulence factors very different than those in
the normal flora. Perhaps strong consideration should not be given to pre-emptively
treating all premature babies with antibiotics for 3 days pending culture results
as is so commonly done. Obviously, the risk of sepsis needs to be carefully weighed
against the likelihood of altering the bacterial microenvironment in each individual
baby. Some bacteria cultured from babies with NEC have been found to have the capability
to inhibit adherence of normal gut flora to CaCo-2 cells in culture.
92
Other studies have shown that certain bacteria isolated from neonates with NEC have
a highly efficient capability to ferment lactose in the presence of hydrolytic products
of casein.
26
These bacteria in turn were found to have the capability to produce severe intestinal
damage in isolated rabbit intestinal loops. When isolated from the original bacterium
and cloned into an otherwise nonpathogenic bacterium, the previously nonpathogenic
bacterium becomes a rapid lactose fermenter and is able to cause disease in the isolated
rabbit intestinal loops. There are likely to be numerous other virulence traits possessed
by NEC-associated bacteria that have not yet been identified. Identification of these
traits, proof of their pathogenicity, and subsequent production of neither active
nor passive immunity against them offer the likelihood of specific prophylaxis against
NEC.
The finding that human milk is likely to be protective against NEC has stimulated
the search for various immunologic and nonimmunologic components of human milk that
could be responsible. A specific nutrient, such as glutamine or nucleotides, may be
involved. Glutamine is one of the most important metabolic substrates for the GI tract.
This amino acid has been found to attenuate various forms of enterocolitis in animal
models and to decrease permeability to lactulose and mannitol in adults. It is thought
to become an essential amino acid during times of stress. It is not normally supplied
to these babies in appreciable quantities by either the enteral or parenteral route
because most premature neonates are not provided with appreciable enteral nutrition,
and neonatal TPN solutions do not contain glutamine. Other growth factors and hormones
that are known to directly improve GI function and maturity, such as epidermal growth
factor, insulin-like growth factor 1, and thyroid hormone, also offer a fertile area
for future investigation for the prevention of NEC.
Understanding the specific steps in the inflammatory cascade resulting in NEC also
should offer opportunities for further intervention. Inhibitors of PAF, stimulators
or inhibitors of nitric oxide synthase, cytokines, Prostaglandins, and leukotrienes
may have a role in the future prevention of NEC. Although intervening in the inflammatory
cascade seems to offer hope, the disease may be so far advanced that rescue at this
stage is no longer likely, and prevention may be impossible. Intervention into more
proximal events in the pathologic cascade by altering the lumenal milieu by immunizing
against microbial virulence factors or maturing the intestinal barrier should offer
the most promise for prophylaxis against NEC.
Related collections
Most cited references109
- Record: found
- Abstract: found
- Article: not found
Translocation of certain indigenous bacteria from the gastrointestinal tract to the mesenteric lymph nodes and other organs in a gnotobiotic mouse model.
A W Garlington, Paul R. Berg (1979)
- Record: found
- Abstract: found
- Article: not found
Development and differences of intestinal flora in the neonatal period in breast-fed and bottle-fed infants.
Matthew Fujita, H Yoshioka, K Iseki (1983)
- Record: found
- Abstract: found
- Article: not found
Breast milk and neonatal necrotising enterocolitis.
A. A. Lucas, T. J. Cole (2015)
