- Record: found
- Abstract: found
- Article: found
Specific gyrA gene mutations predict poor treatment outcome in MDR-TB

Read this article at
Abstract
Objectives
Mutations in the gyrase genes cause fluoroquinolone resistance in Mycobacterium tuberculosis. However, the predictive value of these markers for clinical outcomes in patients with MDR-TB is unknown to date. The objective of this study was to determine molecular markers and breakpoints predicting second-line treatment outcomes in M. tuberculosis patients treated with fourth-generation fluoroquinolones.
Methods
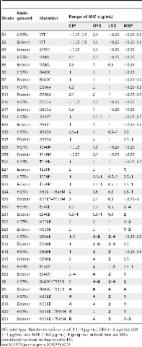
We analysed treatment outcome data in relation to the gyrA and gyrB sequences and MICs of ofloxacin, gatifloxacin and moxifloxacin for pretreatment M. tuberculosis isolates from 181 MDR-TB patients in Bangladesh whose isolates were susceptible to injectable drugs.
Results
The gyrA 90Val, 94Gly and 94Ala mutations were most frequent, with the highest resistance levels for 94Gly mutants. Increased pretreatment resistance levels (>2 mg/L), related to specific mutations, were associated with lower cure percentages, with no cure in patients whose isolates were resistant to gatifloxacin at 4 mg/L. Any gyrA 94 mutation, except 94Ala, predicted a significantly lower proportion of cure compared with all other gyrA mutations taken together (all non-94 mutants + 94Ala) [OR = 4.3 (95% CI 1.4–13.0)]. The difference in treatment outcome was not explained by resistance to the other drugs.
Related collections
Most cited references38
- Record: found
- Abstract: found
- Article: not found
Resistance to fluoroquinolones and second-line injectable drugs: impact on multidrug-resistant TB outcomes.
- Record: found
- Abstract: found
- Article: not found
Outpatient gatifloxacin therapy and dysglycemia in older adults.
- Record: found
- Abstract: found
- Article: found