- Record: found
- Abstract: found
- Article: found
High-Fat Diet-Induced Mitochondrial Dysfunction Promotes Genioglossus Injury – A Potential Mechanism for Obstructive Sleep Apnea with Obesity
Read this article at
Abstract
Purpose
Obesity is a worldwide metabolic disease and a critical risk factor for several chronic conditions. Obstructive sleep apnea (OSA) is an important complication of obesity. With the soaring morbidity of obesity, the prevalence of OSA has markedly increased. However, the underlying mechanism of the high relevance between obesity and OSA has not been elucidated. This study investigated the effects of obesity on the structure and function of the genioglossus to explore the possible mechanisms involved in OSA combined with obesity.
Methods
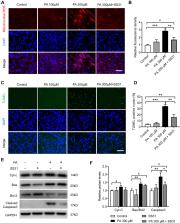
Six-week-old male C57BL/6J mice were fed high-fat diet (HFD, 60% energy) or normal diet (Control, 10% energy) for 16 weeks. The muscle fibre structure and electromyography (EMG) activity of genioglossus were measured. The ultrastructure and function of mitochondrial, oxidative damage and apoptosis in genioglossus were detected by transmission electron microscopy (TEM), qPCR, Western blotting, immunohistochemistry and TUNEL staining. We further studied the influence of palmitic acid (PA) on the proliferation and myogenic differentiation of C2C12 myoblasts, as well as mitochondrial function, oxidative stress, and apoptosis in C2C12 myotubes.
Results
Compared with the control, the number of muscle fibres was decreased, the fibre type was remarkably changed, and the EMG activity had declined in genioglossus. In addition, a HFD also reduced mitochondria quantity and function, induced excessive oxidative stress and increased apoptosis in genioglossus. In vitro, PA treatment significantly inhibited the proliferation and myogenic differentiation of C2C12 myoblasts. Moreover, PA decreased the mitochondrial membrane potential, upregulated mitochondrial reactive oxygen species (ROS) levels, and activated the mitochondrial-related apoptotic pathway in myotubes.
Related collections
Most cited references49
- Record: found
- Abstract: found
- Article: not found
Mitochondria as multifaceted regulators of cell death
- Record: found
- Abstract: found
- Article: not found
Increased prevalence of sleep-disordered breathing in adults.
- Record: found
- Abstract: found
- Article: not found