- Record: found
- Abstract: found
- Article: found
Lactonase Activity and Lipoprotein-Phospholipase A 2 as Possible Novel Serum Biomarkers for the Differential Diagnosis of Autism Spectrum Disorders and Rett Syndrome: Results from a Pilot Study
Read this article at
Abstract
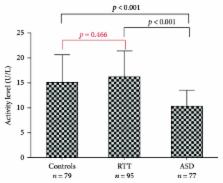
Rett syndrome (RTT) and autism spectrum disorders (ASDs) are not merely expression of brain dysfunction but also reflect the perturbation of physiological/metabolic homeostasis. Accordingly, both disorders appear to be associated with increased vulnerability to toxicants produced by redox imbalance, inflammation, and pollution, and impairment of systemic-detoxifying agents could play a role in the exacerbation of these detrimental processes. To check this hypothesis, the activities of two mechanistically related blood-based enzymes, paraoxonase-1 (arylesterase, paraoxonase, and lactonase), and lipoprotein-associated phospholipase A 2 (Lp-PLA 2) were measured in the serum of 79 ASD and 95 RTT patients, and 77 controls. Lactonase and Lp-PLA 2 showed a similar trend characterized by significantly lower levels of both activities in ASD compared to controls and RTT ( p < 0.001 for all pairwise comparisons). Noteworthy, receiving operator curve (ROC) analysis revealed that lactonase and, mostly, Lp-PLA 2 were able to discriminate between ASD and controls (lactonase: area under curve, AUC = 0.660; Lp-PLA 2, AUC = 0.780), and, considering only females, between ASD and RTT (lactonase, AUC = 0.714; Lp-PLA 2, AUC = 0.881). These results suggest that lactonase and, especially, Lp-PLA 2 activities might represent novel candidate biomarkers for ASD.
Related collections
Most cited references41
- Record: found
- Abstract: found
- Article: not found
Rett syndrome: revised diagnostic criteria and nomenclature.
- Record: found
- Abstract: found
- Article: not found
Metabolic biomarkers of increased oxidative stress and impaired methylation capacity in children with autism.
- Record: found
- Abstract: found
- Article: not found