- Record: found
- Abstract: found
- Article: found
Consolidation treatment for high risk solid tumors in children with myeloablative chemotherapy and autologous hematopoietic progenitor stem cell transplantation
Read this article at
Abstract
Background
In childhood cancer, consolidation treatment with chemotherapy followed by autologous hematopoietic progenitor stem cell transplantation is currently an accepted treatment modality in patients with high-risk solid tumors or in patients who have relapsed after conventional treatment.
Objectives
The objective of this study was to describe the results of transplantation of a group of children who had high-risk solid tumors or relapsed after conventional chemotherapy regimens.
Methods
A retrospective analysis was conducted from January 1998 to October 2004 of all children with pathologic diagnoses of high-risk solid tumors or children that had previously relapsed after conventional chemotherapy and that were subsequently submitted to autologous hematopoietic progenitor stem cell transplantation. The analysis included overall survival rates, event-free survival rates, mortality rates and chemotherapy complications.
Results
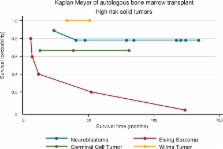
Nineteen patients were submitted to this approach. The age range was from 27 to 196 months with a median age of 52 months. The overall survival rate at 100 days was observed in 79%, the three-year event-free survival rate was 63%. The mortality rate secondary to the myeloablative chemotherapy regimen was 21% (n = 4). Only three patients (15.8%) relapsed with tumor progression after transplant.
Related collections
Most cited references86
- Record: found
- Abstract: found
- Article: not found
Allogeneic and autologous transplantation for haematological diseases, solid tumours and immune disorders: definitions and current practice in Europe.
- Record: found
- Abstract: found
- Article: not found
28 years of high-dose therapy and SCT for neuroblastoma in Europe: lessons from more than 4000 procedures.
- Record: found
- Abstract: found
- Article: not found