- Record: found
- Abstract: found
- Article: found
Dihydromyricetin inhibits cell proliferation, migration, invasion and promotes apoptosis via regulating miR-21 in Human Cholangiocarcinoma Cells
Read this article at
Abstract
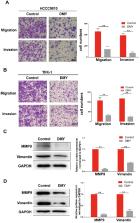
Dihydromyricetin, the most abundant natural flavonoid isolated from Ampelopsis grossedentata, exhibits broad anti-tumor effects. However, the effects of dihydromyricetin on cholangiocarcinoma remain unclear. This study examined the anti-tumor effects of dihydromyricetin in two human cholangiocarcinoma cell lines HCCC9810 and TFK-1, and the underlying mechanism was also investigated. Our study was the first to show that dihydromyricetin significantly inhibited cell proliferation, migration, invasion and promoted apoptosis in cholangiocarcinoma cells. By analyzing the TCGA dataset, we found that expression of miR-21, an oncogene and a potential target of anticancer drugs for cholangiocarcinoma, was upregulated in cholangiocarcinoma tissues compared to paired control tissues. Moreover, dihydromyricetin significantly reduced the expression of miR-21 in a dose-dependent manner. Overexpression of miR-21 remarkably abolished the inhibitory effects of dihydromyricetin on cell proliferation, migration, invasion and abrogated its effect of promoting cell apoptosis in both HCCC9810 and TFK-1 cells. Dihydromyricetin remarkably increased the expression of PTEN and decreased the expression of phosphorylated Akt, while overexpression of miR-21 abrogated the modulation of PTEN/ Akt pathway by dihydromyricetin. Taken together, our study demonstrates that dihydromyricetin inhibits cell proliferation, migration, invasion and promotes apoptosis in cholangiocarcinoma cells via regulating miR-21.
Related collections
Most cited references20
- Record: found
- Abstract: found
- Article: not found
Targeting apoptosis pathways in cancer therapy.
- Record: found
- Abstract: found
- Article: not found
MicroRNA-34a Promotes Renal Fibrosis by Downregulation of Klotho in Tubular Epithelial Cells
- Record: found
- Abstract: not found
- Article: not found