- Record: found
- Abstract: found
- Article: found
New DAG and cAMP Sensors Optimized for Live-Cell Assays in Automated Laboratories
research-article
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
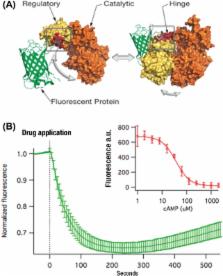
Protein-based, fluorescent biosensors power basic research on cell signaling in health and disease, but their use in automated laboratories is limited. We have now created two live-cell assays, one for diacyl glycerol and another for cAMP, that are robust (Z′ > 0.7) and easily deployed on standard fluorescence plate readers. We describe the development of these assays, focusing on the parameters that were critical for optimization, in the hopes that the lessons learned can be generalized to the development of new biosensor-based assays.
Related collections
Most cited references24
- Record: found
- Abstract: found
- Article: not found
A bright monomeric green fluorescent protein derived from Branchiostoma lanceolatum
Nathan Shaner, Gerard Lambert, Andrew Chammas … (2013)
- Record: found
- Abstract: found
- Article: not found
Optimization of a GCaMP calcium indicator for neural activity imaging.
Jasper Akerboom, Tsai-Wen Chen, Trevor J. Wardill … (2012)
- Record: found
- Abstract: found
- Article: not found
An optimized fluorescent probe for visualizing glutamate neurotransmission
Jonathan S. Marvin, Bart G. Borghuis, Lin Tian … (2013)