- Record: found
- Abstract: found
- Article: found
Plastin and spectrin cooperate to stabilize the actomyosin cortex during cytokinesis
Read this article at
Summary
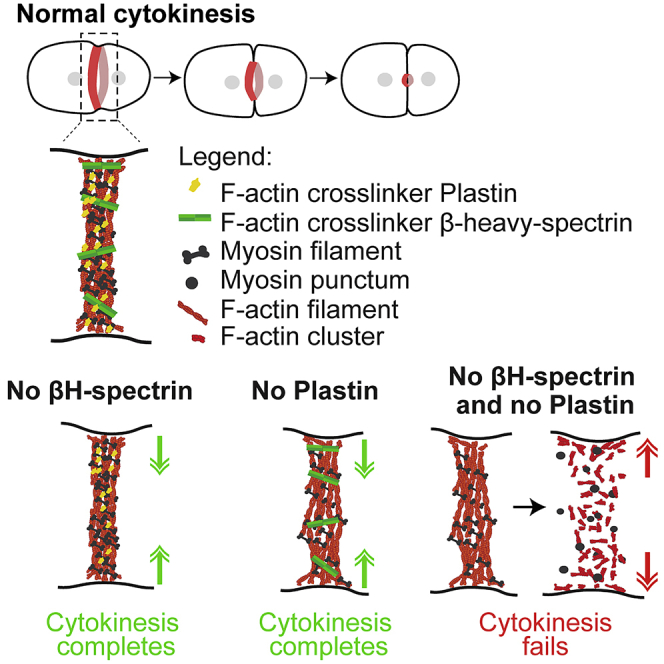
Cytokinesis, the process that partitions the mother cell into two daughter cells, requires the assembly and constriction of an equatorial actomyosin network. Different types of non-motor F-actin crosslinkers localize to the network, but their functional contribution remains poorly understood. Here, we describe a synergy between the small rigid crosslinker plastin and the large flexible crosslinker spectrin in the C. elegans one-cell embryo. In contrast to single inhibitions, co-inhibition of plastin and the βH-spectrin (SMA-1) results in cytokinesis failure due to progressive disorganization and eventual collapse of the equatorial actomyosin network. Cortical localization dynamics of non-muscle myosin II in co-inhibited embryos mimic those observed after drug-induced F-actin depolymerization, suggesting that the combined action of plastin and spectrin stabilizes F-actin in the contractile ring. An in silico model predicts that spectrin is more efficient than plastin at stabilizing the ring and that ring formation is relatively insensitive to βH-spectrin length, which is confirmed in vivo with a sma-1 mutant that lacks 11 of its 29 spectrin repeats. Our findings provide the first evidence that spectrin contributes to cytokinesis and highlight the importance of crosslinker interplay for actomyosin network integrity.
Graphical abstract
Highlights
-
•
Single inhibitions of F-actin crosslinkers do not lead to cytokinesis failure
-
•
Cytokinesis fails upon double inhibition of the crosslinkers plastin and βH-spectrin
-
•
Their joint loss collapses the actomyosin network that forms the cytokinetic ring
-
•
Ring assembly with these two distinct crosslinker types is modeled in silico
Abstract
Sobral, Chan et al. address how F-actin crosslinkers contribute to cytokinesis. Cooperation between the small/rigid crosslinker plastin and the large/flexible crosslinker βH-spectrin is shown to be essential for organization and stability of the equatorial actomyosin network that forms the cytokinetic ring.
Related collections
Most cited references66
- Record: found
- Abstract: found
- Article: not found
Fiji: an open-source platform for biological-image analysis.
- Record: found
- Abstract: not found
- Article: not found
Enzymatic assembly of DNA molecules up to several hundred kilobases.
- Record: found
- Abstract: found
- Article: not found