- Record: found
- Abstract: found
- Article: found
Shikonin promotes hypertrophic scar repair by autophagy of hypertrophic scar-derived fibroblasts
ABSTRACT
Purpose:
To investigate the Shikonin (SHI) induce autophagy of hypertrophic scar-derived fibroblasts (HSFs) and the mechanism of which in repairing hypertrophic scar.
Methods:
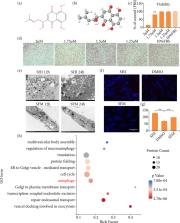
This study showed that SHI induced autophagy from HSFs and repaired skin scars through the AMPK/mTOR pathway. Alamar Blue and Sirius red were used to identify cell activity and collagen. Electron microscopy, label-free quantitative proteomic analysis, fluorescence and other methods were used to identify autophagy. The differences in the expression of autophagy and AMPK/mTOR pathway-related proteins after SHI treatment were quantitatively analyzed by Western blots. A quantitative real-time polymerase chain reaction assay was used to detect the expression of LC3, AMPK and ULK after adding chloroquine (CQ) autophagy inhibitor.
Results:
After treatment with SHI for 24 hours, it was found that the viability of HSFs was significantly reduced, the protein expression of LC3-II/LC3-I and Beclin1 increased, while the protein expression of P62 decreased. The expression of phosphorylated AMPK increased and expression of phosphorylated mTOR decreased. After the use of CQ, the cell autophagy caused by SHI was blocked. The key genes LC3 and P62 were then reexamined by immunohistochemistry using a porcine full-thickness burn hypertrophic scar model, and the results verified that SHI could induce autophagy in vivo.
Related collections
Most cited references44
- Record: found
- Abstract: found
- Article: not found
AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1.
- Record: found
- Abstract: found
- Article: not found
Autophagy: renovation of cells and tissues.
- Record: found
- Abstract: found
- Article: not found