- Record: found
- Abstract: found
- Article: found
Neuroprotective effect of therapeutic hypothermia versus standard care alone after convulsive status epilepticus: protocol of the multicentre randomised controlled trial HYBERNATUS
Read this article at
Abstract
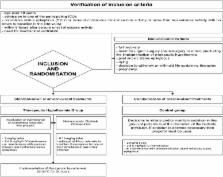
Convulsive status epilepticus (CSE) is a major medical emergency associated with a 50 % morbidity rate. CSE guidelines have recommended prompt management for many years, but there is no evidence to date that they have significantly improved practices or outcomes. Developing neuroprotective strategies for use after CSE holds promise for diminishing morbidity and mortality rates. Hypothermia has been shown to afford neuroprotection in various health conditions. We therefore designed a trial to determine whether 90-day outcomes in mechanically ventilated patients with CSE requiring management in the intensive care unit (ICU) are improved by early therapeutic hypothermia (32–34 °C) for 24 h with propofol sedation. We are conducting a multicentre, open-label, parallel-group, randomised, controlled trial (HYBERNATUS) of potential neuroprotective effects of therapeutic hypothermia and routine propofol sedation started within 8 h after CSE onset in ICU patients requiring mechanical ventilation. Included patients are allocated to receive therapeutic hypothermia (32–34 °C) plus standard care or standard care alone. We plan to enrol 270 patients in 11 ICUs. An interim analysis is scheduled after the inclusion of 135 patients. The main study objective is to evaluate the effectiveness of therapeutic hypothermia (32–34 °C) for 24 h in diminishing 90-day morbidity and mortality (defined as a Glasgow Outcome Scale score <5). The HYBERNATUS trial is expected to a decreased proportion of patients with a Glasgow Outcome Scale score lower than 5 after CSE requiring ICU admission and mechanical ventilation.
Trial registration Clinicaltrials.gov identifier NCT01359332 (registered on 23 May 2011)
Related collections
Most cited references39
- Record: found
- Abstract: found
- Article: not found
Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage.
- Record: found
- Abstract: found
- Article: not found
Hypothermia for Intracranial Hypertension after Traumatic Brain Injury.
- Record: found
- Abstract: found
- Article: not found