- Record: found
- Abstract: found
- Article: found
Enhanced Expression of IGFBP-3 Reduces Radiosensitivity and Is Associated with Poor Prognosis in Oral Squamous Cell Carcinoma
Read this article at
Abstract
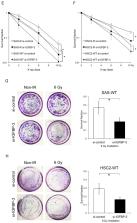
Insulin-like growth factor (IGF) binding protein-3 (IGFBP-3) modulates various cell functions through IGF-dependent or independent mechanisms. However, its biological roles in the radiosensitivity of oral squamous cell carcinoma (OSCC) remain largely unknown. The purpose of this study was to determine the clinical significance and molecular mechanisms of the association between IGFBP-3 and OSCC radiosensitivity. We performed an immunohistochemical analysis of IGFBP-3 in 52 OSCC specimens from patients treated with preoperative chemoradiotherapy and surgery (phase II study). Associations between IGFBP-3 expression and clinicopathological features were also evaluated. In addition, we examined the effects of IGFBP-3 on post-X-ray irradiation radiosensitivity and DNA damage in vitro. High IGFBP-3 expression was significantly correlated with poor chemoradiotherapy responses and prognosis. With IGFBP-3 knockdown, irradiated OSCC cells exhibited significantly higher radiosensitivity compared with that of control cells. Moreover, IGFBP-3 depletion in OSCC cells reduced phosphorylation of the DNA-dependent protein kinase catalytic subunit (DNA-PKcs), which is required for DNA double-strand break repair during non-homologous end joining. These findings indicate that IGFBP-3 may have a significant role in regulating DNA repair and is be a potential biomarker for predicting clinical response to radiotherapy and prognosis in OSCC.
Related collections
Most cited references37
- Record: found
- Abstract: found
- Article: not found
IGF binding proteins in cancer: mechanistic and clinical insights.
- Record: found
- Abstract: found
- Article: not found
Chromosomal stability and the DNA double-stranded break connection.
- Record: found
- Abstract: found
- Article: found