- Record: found
- Abstract: found
- Article: found
Bullous Pemphigoid Associated With COVID-19 Vaccines: An Italian Multicentre Study
Read this article at
Abstract
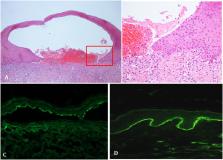
Bullous pemphigoid (BP) is an autoimmune bullous disease caused by circulating autoantibodies toward the hemidesmosomal antigens BP180 and BP230. Cases of BP have been described following vaccinations against tetanus, poliomyelitis, diphtheria, influenza, pneumococcus, meningococcus, hepatitis B and rabies. The putative mechanism by which COVID-19-vaccines may induce BP has not been clarified. An Italian multicentre study was conducted to collect clinical, histopathological and immunopathological data of patients with BP associated with COVID-19-vaccines. Twenty-one cases were collected, including 9 females and 12 males (M/F = 1.3) with a median age at diagnosis of 82 years. Seventeen patients received the COMIRNATY Pfizer-BioNTech vaccine, two the Moderna mRNA-1273 vaccine, one the ChAdOx1/nCoV-19-AstraZeneca/ Vaxzevria vaccine and one received the first dose with the ChAdOx1/nCoV-19-AstraZeneca/Vaxzevria vaccine and the second dose with the COMIRNATY Pfizer-BioNTech vaccine. Median latency time between the first dose of anti-SARS-CoV-2 vaccine and the onset of cutaneous manifestations was 27 days. Median BPDAI at onset was 42. Eleven out of seventeen patients (65%) had positive titres for anti-BP180 antibodies with a median value of 106.3 U/mL on ELISA; in contrast, only five out of seventeen (29%) were positive for anti-BP230 antibodies, with a median of 35.3 U/mL. In conclusion, in terms of mean age, disease severity at diagnosis and clinical phenotype vaccine-associated BP patients seem to be similar to idiopathic BP with an overall benign course with appropriate treatment. On the other hand, the slight male predominance and the reduced humoral response to BP230 represent peculiar features of this subset of patients.
Related collections
Most cited references40
- Record: found
- Abstract: found
- Article: not found
Cutaneous Reactions Reported after Moderna and Pfizer COVID-19 Vaccination: A Registry-Based Study of 414 Cases
- Record: found
- Abstract: found
- Article: found
Reaction of Human Monoclonal Antibodies to SARS-CoV-2 Proteins With Tissue Antigens: Implications for Autoimmune Diseases

- Record: found
- Abstract: found
- Article: found