- Record: found
- Abstract: found
- Article: found
Generation of orthotopic patient-derived xenograft models for pancreatic cancer using tumor slices

Read this article at
Summary
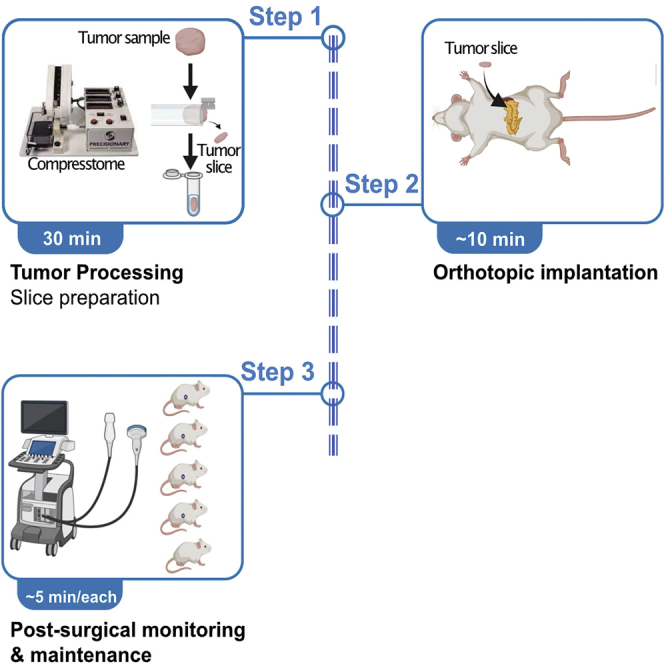
Orthotopic patient-derived xenograft models recapitulate the genomic complexity of the original tumor and some aspects of local microenvironment, useful for rapid development and validation of personalized treatment strategies. Here, we precisely describe a protocol for generating tumor slices from human or murine-derived pancreatic cancer. They are then implanted directly into the murine pancreas, monitored using ultrasound, with a 90% success rate. This assay creates a clinically relevant in vivo model facilitating personalized treatment development.
Graphical abstract
Highlights
-
•
A highly efficient protocol for patient-derived xenograft (PDX) mouse model generation
-
•
Tumor slice preparation from human or murine-derived pancreatic tumor
-
•
Direct orthotopic implantations of tumor slices into mouse pancreas
-
•
Tumor slices, compared to cell lines or fragments, improves engraftment to about 90%
Abstract
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
Abstract
Orthotopic patient-derived xenograft models recapitulate the genomic complexity of the original tumor and some aspects of local microenvironment, useful for rapid development and validation of personalized treatment strategies. Here, we precisely describe a protocol for generating tumor slices from human or murine-derived pancreatic cancer. They are then implanted directly into the murine pancreas, monitored using ultrasound, with a 90% success rate. This assay creates a clinically relevant in vivo model facilitating personalized treatment development.
Related collections
Most cited references4
- Record: found
- Abstract: found
- Article: not found
Development of orthotopic pancreatic tumor mouse models.
- Record: found
- Abstract: found
- Article: not found
Barriers to generating PDX models of HPV-related head and neck cancer.
- Record: found
- Abstract: found
- Article: not found