- Record: found
- Abstract: found
- Article: not found
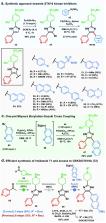
Modular Synthesis of Di- and Trisubstituted Imidazoles from Ketones and Aldehydes: A Route to Kinase Inhibitors
Read this article at
Abstract

A one-pot and modular approach to the synthesis of 2,4(5)-disubstituted imidazoles was developed based on ketone oxidation, employing catalytic HBr and DMSO, followed by imidazole condensation with aldehydes. This methodology afforded twenty-nine disubstituted NH-imidazoles (23%–85% yield). A three-step synthesis of 20 kinase inhibitors was achieved by employing this oxidation–condensation protocol, followed by bromination and Suzuki coupling in the imidazole ring to yield trisubstituted NH-imidazoles (23%–69%, three steps). This approach was also employed in the synthesis of known inhibitor GSK3037619A.
Related collections
Most cited references37
- Record: found
- Abstract: found
- Article: not found
Comprehensive characterization of the Published Kinase Inhibitor Set.
- Record: found
- Abstract: found
- Article: not found
