- Record: found
- Abstract: found
- Article: found
Simple modeling of familial Alzheimer’s disease using human pluripotent stem cell-derived cerebral organoid technology
Read this article at
Abstract
Background
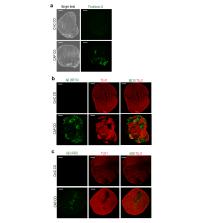
Cerebral organoids (COs) are the most advanced in vitro models that resemble the human brain. The use of COs as a model for Alzheimer’s disease (AD), as well as other brain diseases, has recently gained attention. This study aimed to develop a human AD CO model using normal human pluripotent stem cells (hPSCs) that recapitulates the pathological phenotypes of AD and to determine the usefulness of this model for drug screening.
Methods
We established AD hPSC lines from normal hPSCs by introducing genes that harbor familial AD mutations, and the COs were generated using these hPSC lines. The pathological features of AD, including extensive amyloid-β (Aβ) accumulation, tauopathy, and neurodegeneration, were analyzed using enzyme-linked immunosorbent assay, Amylo-Glo staining, thioflavin-S staining, immunohistochemistry, Bielschowsky’s staining, and western blot analysis.
Results
The AD COs exhibited extensive Aβ accumulation. The levels of paired helical filament tau and neurofibrillary tangle-like silver deposits were highly increased in the AD COs. The number of cells immunoreactive for cleaved caspase-3 was significantly increased in the AD COs. In addition, treatment of AD COs with BACE1 inhibitor IV, a β-secretase inhibitor, and compound E, a γ-secretase inhibitor, significantly attenuated the AD pathological features.
Related collections
Most cited references54
- Record: found
- Abstract: found
- Article: not found
Cerebral organoids model human brain development and microcephaly
- Record: found
- Abstract: found
- Article: not found
The antibody aducanumab reduces Aβ plaques in Alzheimer's disease.
- Record: found
- Abstract: found
- Article: not found