- Record: found
- Abstract: found
- Article: found
Differences in PGE 2 Production between Primary Human Monocytes and Differentiated Macrophages: Role of IL-1β and TRIF/IRF3
Read this article at
Abstract
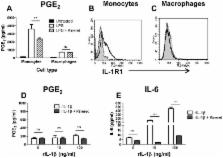
Prostaglandin E2 (PGE 2) is induced in vivo by bacterial products including TLR agonists. To determine whether PGE 2 is induced directly or via IL-1β, human monocytes and macrophages were cultured with LPS or with Pam3CSK4 in presence of caspase-1 inhibitor, ZVAD, or IL-1R antagonist, Kineret. TLR agonists induced PGE 2 in macrophages exclusively via IL-1β-independent mechanisms. In contrast, ZVAD and Kineret reduced PGE 2 production in LPS-treated (but not in Pam3CSK4-treated) monocytes, by 30–60%. Recombinant human IL-1β augmented COX-2 and mPGES-1 mRNA and PGE 2 production in LPS-pretreated monocytes but not in un-primed or Pam3CSK4-primed monocytes. This difference was explained by the finding that LPS but not Pam3CSK4 induced phosphorylation of IRF3 in monocytes suggesting activation of the TRIF signaling pathway. Knocking down TRIF, TRAM, or IRF3 genes by siRNA inhibited IL-1β-induced COX-2 and mPGES-1 mRNA. Blocking of TLR4 endocytosis during LPS priming prevented the increase in PGE 2 production by exogenous IL-1β. Our data showed that TLR2 agonists induce PGE 2 in monocytes independently from IL-1β. In the case of TLR4, IL-1β augments PGE 2 production in LPS-primed monocytes (but not in macrophages) through a mechanism that requires TLR4 internalization and activation of the TRIF/IRF3 pathway. These findings suggest a key role for blood monocytes in the rapid onset of fever in animals and humans exposed to bacterial products and some novel adjuvants.
Related collections
Most cited references39
- Record: found
- Abstract: found
- Article: not found
IRFs: master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors.
- Record: found
- Abstract: not found
- Article: not found
Rapid detection of octamer binding proteins with 'mini-extracts', prepared from a small number of cells.
- Record: found
- Abstract: found
- Article: not found